J Korean Neurosurg Soc.
2015 Feb;57(2):82-87. 10.3340/jkns.2015.57.2.82.
Impaired Voluntary Wheel Running Behavior in the Unilateral 6-Hydroxydopamine Rat Model of Parkinson's Disease
- Affiliations
-
- 1Department of Neurosurgery, Zhujiang Hospital, Southern Medical University, Guangzhou, China. ruxiangcd@126.com
- 2Key Laboratory on Brain Function Repair and Regeneration of Guangdong, Southern Medical University, Guangzhou, China.
- 3Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences, Beijing, China.
- 4Neuroscience Research Institute of North Carolina, Winston-Salem, NC, USA.
- 5Department of Neurosurgery, The Military General Hospital of Beijing PLA, Beijing, China.
- KMID: 2191187
- DOI: http://doi.org/10.3340/jkns.2015.57.2.82
Abstract
OBJECTIVE
The aim of this study was to investigate voluntary wheel running behavior in the unilateral 6-hydroxydopamine (6-OHDA) rat model.
METHODS
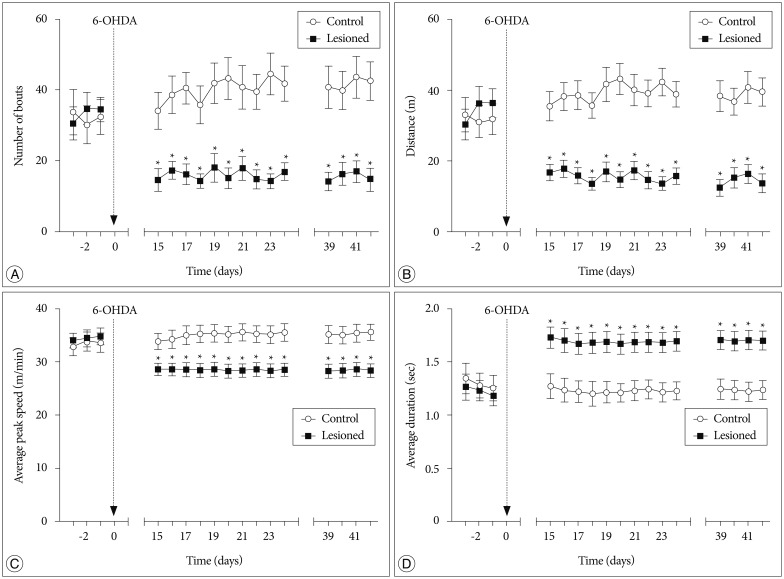
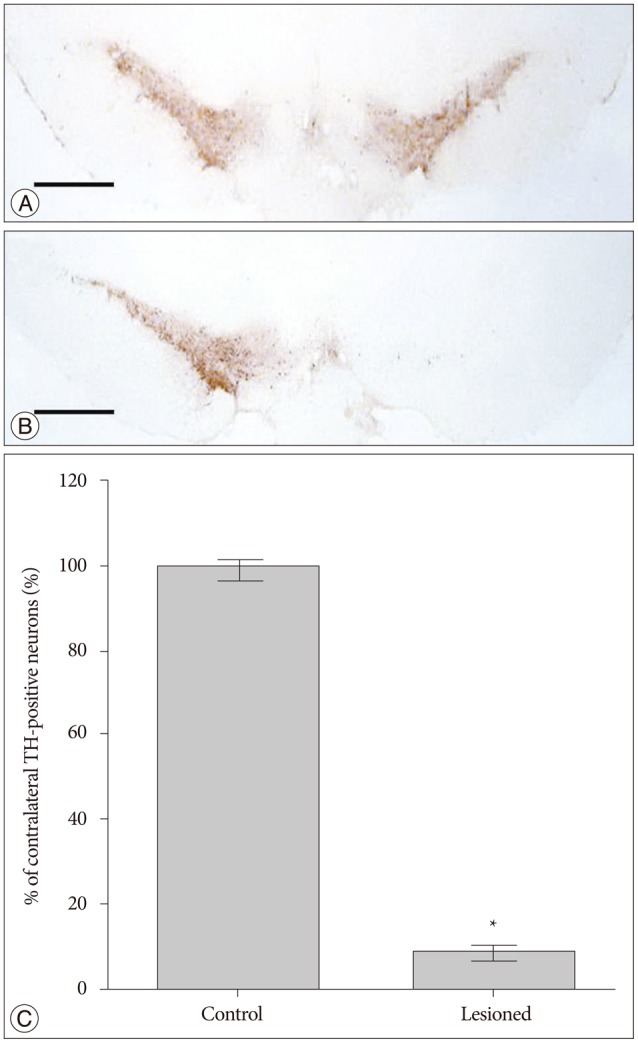
Male Sprague-Dawley rats were assigned to 2 groups : 6-OHDA group (n=17) and control group (n=8). The unilateral 6-OHDA rat model was induced by injection of 6-OHDA into unilateral medial forebrain bundle using a stereotaxic instrument. Voluntary wheel running activity was assessed per day in successfully lesioned rats (n=10) and control rats. Each behavioral test lasted an hour. The following parameters were investigated during behavioral tests : the number of running bouts, the distance moved in the wheel, average peak speed in running bouts and average duration from the running start to the peak speed.
RESULTS
The number of running bouts and the distance moved in the wheel were significantly decreased in successfully lesioned rats compared with control rats. In addition, average peak speed in running bouts was decreased, and average duration from the running start to the peak speed was increased in lesioned animals, which might indicate motor deficits in these rats. These behavioral changes were still observed 42 days after lesion.
CONCLUSION
Voluntary wheel running behavior is impaired in the unilateral 6-OHDA rat model and may represent a useful tool to quantify motor deficits in this model.
Keyword
MeSH Terms
Figure
Reference
-
1. Belke TW, Wagner JP. The reinforcing property and the rewarding aftereffect of wheel running in rats : a combination of two paradigms. Behav Processes. 2005; 68:165–172. PMID: 15686826.
Article2. Bové J, Perier C. Neurotoxin-based models of Parkinson's disease. Neuroscience. 2012; 211:51–76. PMID: 22108613.
Article3. Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control : rewarding, aversive, and alerting. Neuron. 2010; 68:815–834. PMID: 21144997.
Article4. Carman LS, Gage FH, Shults CW. Partial lesion of the substantia nigra : relation between extent of lesion and rotational behavior. Brain Res. 1991; 553:275–283. PMID: 1681983.
Article5. Cenci MA, Whishaw IQ, Schallert T. Animal models of neurological deficits : how relevant is the rat? Nat Rev Neurosci. 2002; 3:574–579. PMID: 12094213.
Article6. Chang JY, Shi LH, Luo F, Woodward DJ. High frequency stimulation of the subthalamic nucleus improves treadmill locomotion in unilateral 6-hydroxydopamine lesioned rats. Brain Res. 2003; 983:174–184. PMID: 12914978.
Article7. Chang JY, Shi LH, Luo F, Zhang WM, Woodward DJ. Studies of the neural mechanisms of deep brain stimulation in rodent models of Parkinson's disease. Neurosci Biobehav Rev. 2008; 32:352–366. PMID: 18035416.
Article8. da Conceição FS, Ngo-Abdalla S, Houzel JC, Rehen SK. Murine model for Parkinson's disease : from 6-OH dopamine lesion to behavioral test. J Vis Exp. 2010; 35:1376. http://dx.doi.org/10.3791/1376. PMID: 20081770.9. Deumens R, Blokland A, Prickaerts J. Modeling Parkinson's disease in rats : an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol. 2002; 175:303–317. PMID: 12061862.
Article10. Fornaguera J, Schwarting RK. Early behavioral changes after nigro-striatal system damage can serve as predictors of striatal dopamine depletion. Prog Neuropsychopharmacol Biol Psychiatry. 1999; 23:1353–1368. PMID: 10631763.
Article11. Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin Neurophysiol. 2008; 119:1459–1474. PMID: 18467168.
Article12. Inden M, Taira T, Kitamura Y, Yanagida T, Tsuchiya D, Takata K, et al. PARK7 DJ-1 protects against degeneration of nigral dopaminergic neurons in Parkinson's disease rat model. Neurobiol Dis. 2006; 24:144–158. PMID: 16860563.
Article13. Ito C, Onodera K, Sakurai E, Sato M, Watanabe T. Effect of cocaine on the histaminergic neuron system in the rat brain. J Neurochem. 1997; 69:875–878. PMID: 9231750.
Article14. Iversen SD, Iversen LL. Dopamine : 50 years in perspective. Trends Neurosci. 2007; 30:188–193. PMID: 17368565.15. Kasahara T, Kubota M, Miyauchi T, Noda Y, Mouri A, Nabeshima T, et al. Mice with neuron-specific accumulation of mitochondrial DNA mutations show mood disorder-like phenotypes. Mol Psychiatry. 523; 11:577–593. PMID: 16619054.
Article16. Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain. 2013; 136(Pt 8):2419–2431. PMID: 23884810.
Article17. Lau YS, Fung YK. Pharmacological effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on striatal dopamine receptor system. Brain Res. 1986; 369:311–315. PMID: 3486026.
Article18. Leng A, Mura A, Hengerer B, Feldon J, Ferger B. Effects of blocking the dopamine biosynthesis and of neurotoxic dopamine depletion with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on voluntary wheel running in mice. Behav Brain Res. 2004; 154:375–383. PMID: 15313025.
Article19. Liebetanz D, Baier PC, Paulus W, Meuer K, Bähr M, Weishaupt JH. A highly sensitive automated complex running wheel test to detect latent motor deficits in the mouse MPTP model of Parkinson's disease. Exp Neurol. 2007; 205:207–213. PMID: 17341420.
Article20. Meijer JH, Robbers Y. Wheel running in the wild. Proc Biol Sci. 2014; 281:20140210. http://dx.doi.org/10.1098/rspb.2014.0210. PMID: 24850923.
Article21. Nakata Y, Yasuda T, Mochizuki H. Recent progress in gene therapy for Parkinson's disease. Curr Mol Med. 2012; 12:1311–1318. PMID: 22834832.
Article22. Niu C, Mei J, Pan Q, Fu X. Nigral degeneration with inclusion body formation and behavioral changes in rats after proteasomal inhibition. Stereotact Funct Neurosurg. 2009; 87:69–81. PMID: 19223692.
Article23. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. ed 6. New York: Academic Press;2007.24. Reglodi D, Lubics A, Tamás A, Szalontay L, Lengvári I. Pituitary adenylate cyclase activating polypeptide protects dopaminergic neurons and improves behavioral deficits in a rat model of Parkinson's disease. Behav Brain Res. 2004; 151:303–312. PMID: 15084446.
Article25. Rhodes JS, Gammie SC, Garland T Jr. Neurobiology of Mice Selected for High Voluntary Wheel-running Activity. Integr Comp Biol. 2005; 45:438–455. PMID: 21676789.
Article26. Rosenwasser AM, Clark JW, Fixaris MC, Belanger GV, Foster JA. Effects of repeated light-dark phase shifts on voluntary ethanol and water intake in male and female Fischer and Lewis rats. Alcohol. 2010; 44:229–237. PMID: 20488643.
Article27. Rosenwasser AM, Fixaris MC. Chronobiology of alcohol : studies in C57BL/6J and DBA/2J inbred mice. Physiol Behav. 110-111:2013; 140–147. PMID: 23313401.28. Sherwin CM. Voluntary wheel running : a review and novel interpretation. Anim Behav. 1998; 56:11–27. PMID: 9710457.29. Simola N, Morelli M, Carta AR. The 6-hydroxydopamine model of Parkinson's disease. Neurotox Res. 2007; 11:151–167. PMID: 17449457.
Article30. Ungerstedt U. Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971; 367:69–93. PMID: 4332693.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Voluntary Wheel Running Improves Spatial Learning Memory by Suppressing Inflammation and Apoptosis via Inactivation of Nuclear Factor Kappa B in Brain Inflammation Rats
- Voluntary Wheel Running Exercise Improves Aging-Induced Sarcopenia via Activation of Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1α/Fibronectin Type III Domain-Containing Protein 5/Adenosine Monophosphate-Activated Protein Kinase Signaling Pathway
- Morphological Changes in Dopaminergic Neurons in Selective Parkinson's Rat Model
- The Significance of Apomorphine-Induced Rotational Behavior in Partial Lesioned Rat Parkinsonian Models with 6-hydroxydopamine
- Genomic Changes in the Striatum of Unilateral 6-hydroxydopamine Lesioned Parkinson Rat Model