J Korean Med Assoc.
2010 Sep;53(9):761-768. 10.5124/jkma.2010.53.9.761.
Advances in clinical trials technologies
- Affiliations
-
- 1Department of Pharmacology, Clinical Pharmacology & Clinical Trials Center Seoul National University, College of Medicine and Seoul National University Hospital, Seoul, Korea. ijjang@snu.ac.kr
- KMID: 2188368
- DOI: http://doi.org/10.5124/jkma.2010.53.9.761
Abstract
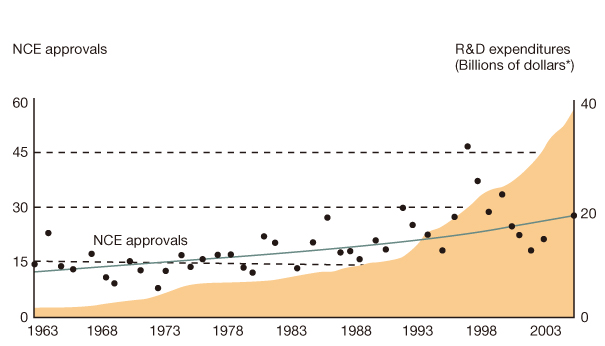
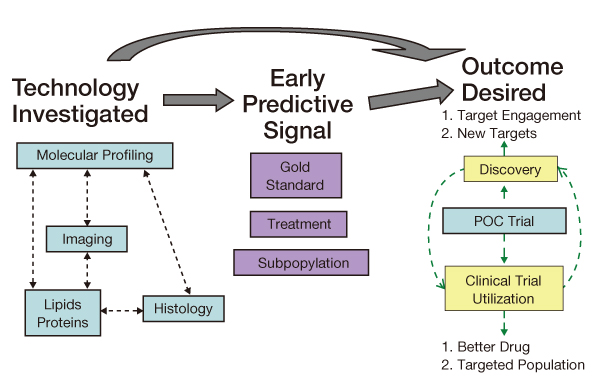
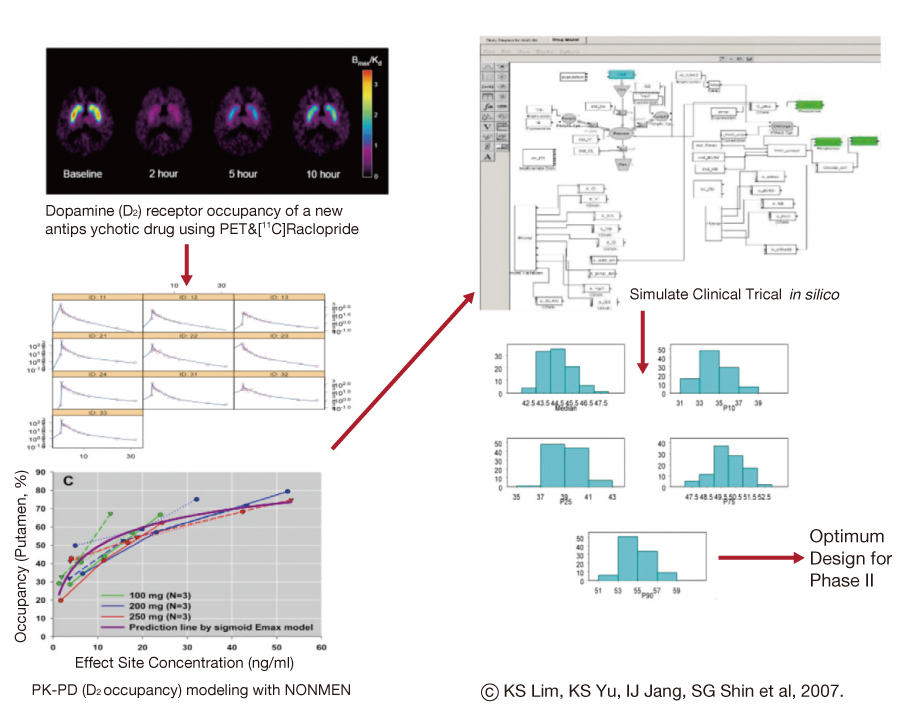
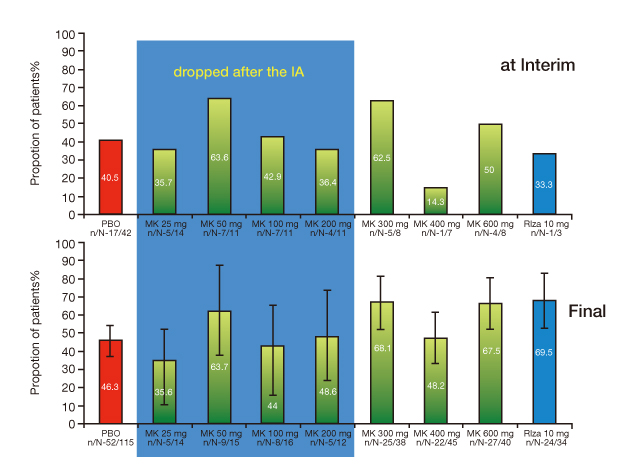
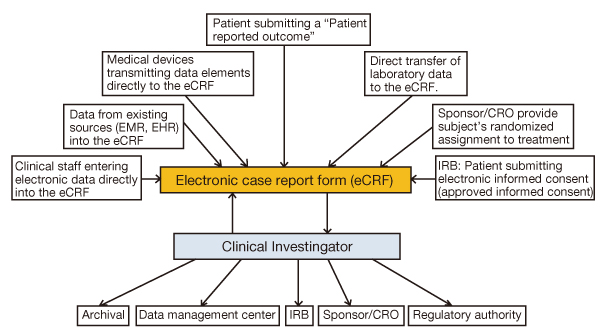
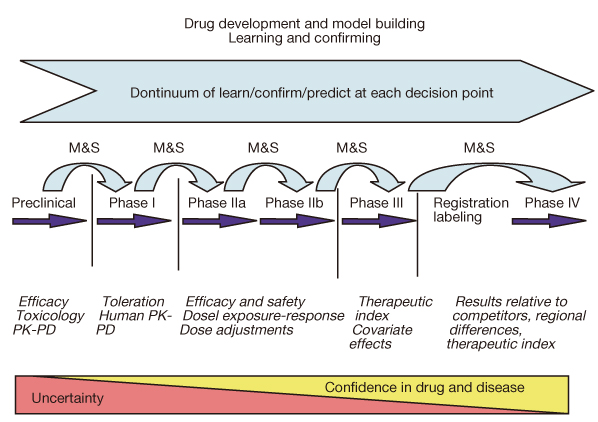
- The reported failure rates in phase 2A and 2B for drugs completing phase 1 and proof of concept (POC) and those in phase 3 for drugs completing phase 2B are as high as 40% and 50%, respectively. These attrition rates are often due to poor design and analysis of data, such as insufficient size and duration for safety, insufficient dose range, neglected time course of drug response, poor analysis of categorical data, and neglected subgroups. Collaborations among industry, academia, and government are becoming more important in developing new clinical trial technology and bridging the gap between the discoveries in basic science and product development for public health. The benefit of using new science are emphasized in a white paper "Critical Path Initiatives" and following reports by United States Food and Drug Administration (US FDA) and consortium activities such as the Critical Path Institute of the US. When properly analyzed, biomarkers of pharmacodynamics (PD), disease progress, or toxicity can reduce failure rates in phase 2 or 3 by providing tools for an early decision of GO or NO-GO, optimal dose range, etc. Eventually this can lead to monitoring tools for outcomes of disease, better and safer therapy, and tailored medicine. In the past, traditional biomarkers were single measures of physiological or biochemical processes such as HIV burden, cancer markers, blood glucose, blood pressure, etc. Now multiple or clusters of omics measures and in vivo imaging are being added to the list. These biomarkers are best utilized when more quantitative model based drug development tools such as modeling and simulation of pharmacokinetics/PD and disease model are implemented in the overall drug development processes. New clinical trial designs such as microdosing and adaptive designs are also useful for the application of biomarkers for efficient clinical trials. Bioinformatics can also facilitate clinical trials with electronic data capturing, data transfer, and so on.
MeSH Terms
Figure
Reference
-
2. Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004. 3:711–715.
Article3. The Critical Path Initiative. Leonard Sacks, Office of Critical Path Programs, FDA. 2010. Washington DC: DIA.5. Wagner JA, Williams SA, Webster CJ. Biomarkers and surrogate end points for fit-forpurpose development and regulatory evaluation of new drugs. Clin Pharmacol Ther. 2007. 81:104–107.
Article6. Baker M. In biomarkers we trust? Nature Biotechnology. 2005. 23:297–304.
Article7. Wong Peggy. Statistical Challenges in Translating Biomarkers into Clinical Utility for Drug Development. 2010. In : 46th DIA Annual Meeting; Washington, DC.8. Food and Drug Administration. Guidance for Industry: Pharmacogenomic Data Submissions. 2005. 03.10. Reflection Paper on Methodological Issues in Confirmatory Clinical Trials with Flexible Design and Analysis Plan. Committee for Medicinal Products for Human Use. 2006. 03. http://www.emea.eu.int/pdfs/human/ewp/245902en.pdf.11. FDA Guidance for Industry. Adaptive Design Clinical Trials for Drugs and Biologics (draft guidance 2010).13. Lappin G, Kuhnz W, Jochemsen R, Kneer J, Chaudhary A, Oosterhuis B, Drijfhout WJ, Rowland M, Garner RC. Use of microdosing to predict pharmacokinetics at the therapeutic dose: experience with 5 drugs. Clin Pharmacol Ther. 2006. 80:203–215.
Article