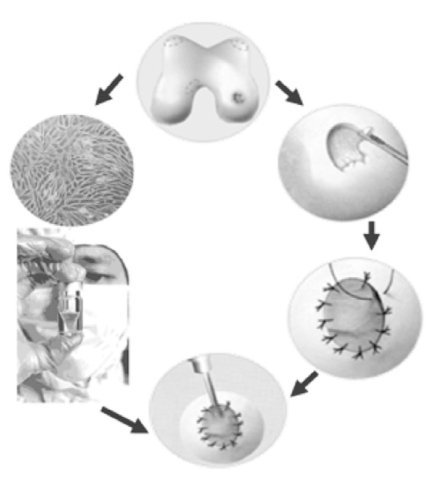
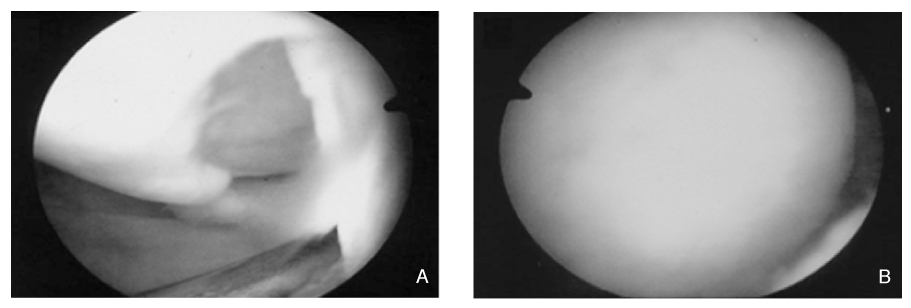
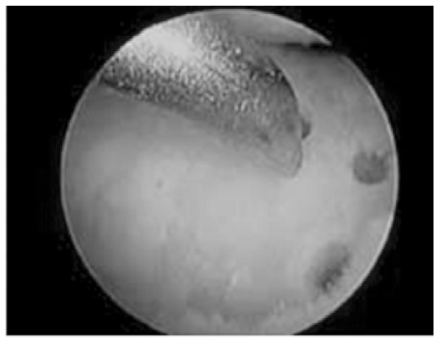
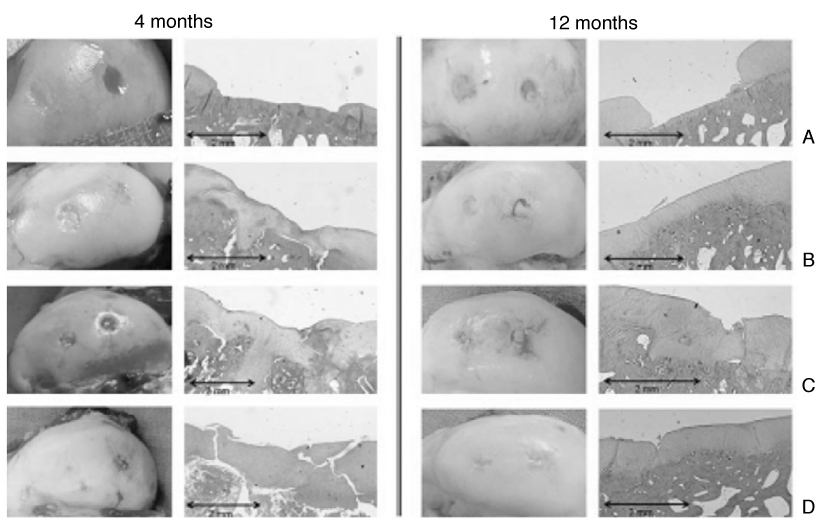
J Korean Med Assoc.
2009 Nov;52(11):1077-1089. 10.5124/jkma.2009.52.11.1077.
Cartilage Repair Using Mesenchymal Stem Cells
- Affiliations
-
- 1Department of Orthopedic Surgery, Ajou University College of Medicine, Korea. bhmin@ajou.ac.kr
- 2Cell Therapy Center, Ajou University Medical Center, Korea.
- KMID: 2188090
- DOI: http://doi.org/10.5124/jkma.2009.52.11.1077
Abstract
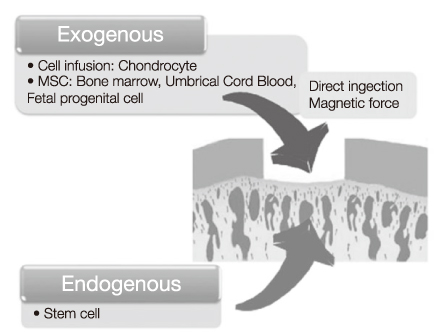
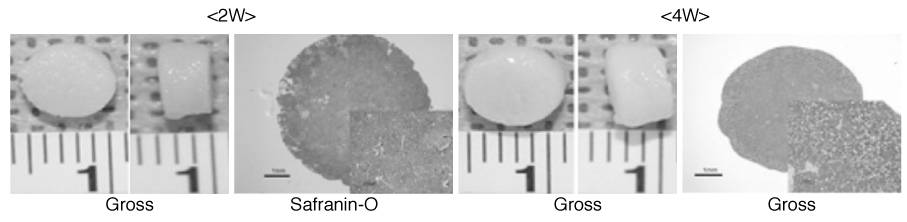
- Articular cartilage defect rarely heals spontaneously due to its avascularity and low cellularity. Even small articular cartilage defects can develop into osteoarthritis, and subsequently, its management has been a major clinical concern. Although there are several treatment options for cartilage defect, no treatment has been established as a gold standard procedure. Bone marrow stimulation techniques which is equivalent to microfracture these days has been adapted as first line treatment, attributed to their technical easiness and minimal invasiveness to patients. However, this procedure has limitation in reproducing hyaline cartilage, so recent cell-based therapies using autologous chondrocytes or mesenchymal stem cells have drawn particular attention. MSCs regardless of its origin have shown significant potential for chondrogenesis. Novel approaches using MSCs as an alternative cell source for patient derived chondrocytes are currently on trial. In this review, stem cells from various origins considered as cell sources and potential application of mesenchymal stem cells to promote cartilage repair will be discussed. While differentiation of stem cell can be well controlled in vitro, it is not easy to predict the course of differentiation when the stem cell is transplanted. Some novel methods using physical stimulation and material based techniques for differentiation control are introduced in this context. Such differentiation control will be beneficial when it is adapted before transplantation. We call it preconditioning.
MeSH Terms
Figure
Reference
-
1. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994. 331:889–895.
Article2. Peterson L, Menche D, Grande D, Klein M, Burmester G, Pugh J, Pitman M. Chondrocyte transplantation-an experimental model in the rabbit. Trans Orthop Res Soc. 1984. 9:218.3. Grande DA, Pitman MI, Peterson L, Menche D, Klein M. The repair of experimentally produced defects in rabbit articular cartilage by autologous chondrocyte transplantation. J Orthop Res. 1989. 7:208–218.
Article4. Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000. 374:212–234.
Article5. Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003. 85-A:Suppl 2. 17–24.6. Steadman JR, Rodkey WG, Briggs KK, Rodrigo J. The microfracture procedure: rationale, technique, and clinical observations for treatment of articular cartilage defects. J Sports Traumatol Relat Res. 1998. 20:61–70.7. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009. 02. 26. [Epub ahead of print].8. Chen H, Sun J, Hoemann CD, Lascau-Coman V, Ouyang W, McKee MD, Shive MS, Buschmann MD. Drilling and microfracture lead to different bone structure and necrosis during bone-marrow stimulation for cartilage repair. J Orthop Res. 2009. 04. 28. [Epub ahead of print].
Article9. Mori S. Bone fracture and the healing mechanisms. Microdamage and microfracture. Clin Calcium. 2009. 19:699–703.10. Kang SW, Bada LP, Kang CS, Lee JS, Kim CH, Park JH, Kim BS. Articular cartilage regeneration with microfracture and hyaluronic acid. Biotechnol lett. 2008. 30:435–439.
Article11. Breinan HA, Martin SD, Hsu HP, Spector M. Healing of canine articular cartilage defects treated with microfracture, a type II collagen matrix, or cultured autologous chondrocytes. J Orthop Res. 2000. 18:781–789.
Article12. Kramer J, Bohrnsen F, Lindner U, Behrens P, Schlenke P, Rohwedel J. In vivo matrix-guided human mesenchymal stem cells. Cell Mol Life Sci. 2006. 63:616–626.
Article13. Dorotka R, Bindreiter U, Macfelda K, Windberger U, Nehrer S. Marrow stimulation and chondrocyte transplantation using a collagen matrix for cartilage repair. Osteoarthritis Cartilage. 2005. 13:655–664.
Article14. Khang G, Kim SH, Kim MS, Rhee JM, Lee HB. Recent and future directions of stem cells for the application of regenerative medicine. Tissue Eng Regen Med. 2007. 4:441–470.15. Min B-H, Kim HJ, Lim H, Park CS, Park SR. Effects of ageing and arthritic disease on nitric oxide production by human articular chondrocytes. Exp Mol Med. 2001. 33:299–302.
Article16. Kim HJ, Park SR, Park HJ, Choi BH, Min B-H. Potential predictive markers for proliferative capacity of cultured human articular chondrocytes: PCNA and p21. Artificial Organs. 2005. 29:393–398.
Article17. De Bari C, Dell'ccio F, Luyten FP. Failure of in vitro differentiated mesenchymal stem cells from the synovial membrane to form ectopic stable cartilage in vivo. Arthritis Rheum. 2004. 50:142–150.
Article18. Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002. 10:199–206.
Article19. Wakitani S, Mitsuoka T, Nakamura N, Toritsuka Y, Nakamura Y, Horibe S. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell Transplant. 2004. 13:595–600.
Article20. Wakitani S, Nawata M, Tensho K, Okabe T, Machida H, Ohgushi H. Repair of articular cartilage defects in the patellofemoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007. 1:74–79.
Article21. Yan H, Yu C. Repair of full-thickness cartilage defects with cells of different origin in a rabbit model. Arthroscopy. 2007. 23:178–187.
Article22. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000. 109:235–242.
Article23. Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H, Weber RM, Ewerbeck V, Richter W. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003. 48:418–429.
Article24. Im GI, Shin YW, Lee KB. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells. Osteoarthritis Cartilage. 2005. 13:845–853.
Article25. Recklies AD, Baillargeon L, White C. Regulation of cartilage oligomeric matrix protein synthesis in human synovial cells and articular chondrocytes. Arthritis Rheum. 1998. 41:997–1006.
Article26. Fife RS, Caterson B, Myers SL. Identification of link proteins in canine synovial cell cultures and canine articular cartilage. J Cell Biol. 1985. 100:1050–1055.
Article27. Hamerman D, Smith C, Keiser HD, Craig R. Glycosaminoglycans produced by human synovial cell cultures. Coll Relat Res. 1982. 2:313–329.
Article28. Miyamoto A, Deie M, Yamasaki T, Nakamae A, Shinomiya R, Adachi N, Ochi M. The role of the synovium in repairing cartilage defects. Knee Surg Sports Traumatol Arthrosc. 2007. 15:1083–1093.
Article29. Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005. 52:2521–2529.
Article30. Mochizuki T, Muneta T, Sakaguchi Y, Nimura A, Yokoyama A, Koga H, Sekiya I. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 2006. 54:843–853.
Article31. Shirasawa S, Sekiya I, Sakaguchi Y, Yagishita K, Ichinose S, Muneta T. In vitro chondrogenesis of human synoviumderived mesenchymal stem cells: optimal condition and comparison with bone marrow-derived cells. J Cell Biochem. 2006. 97:84–87.
Article32. Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007. 327:449–462.
Article33. Peiyz M, Heyz F, Boycey BM, Kishy VL. Repair of full-thickness femoral condyle cartilage defects using allogeneic synovial cell-engineered tissue constructs. Osteoarthritis Cartilage. 2009. 17:714–722.
Article34. Matsumoto T, Kubo S, Meszaros LB, Corsi KA, Cooper GM, Li G, Usas A, Osawa A, Fu FH, Huard J. The influence of sex on the chondrogenic potential of muscle-derived stem cells: implications for cartilage regeneration and repair. Arthritis Rheum. 2008. 58:3809–3819.
Article35. Goldring MB. Are bone morphogenetic proteins effective inducers of cartilage repair? Ex vivo transduction of muscle-derived stem cells. Arthritis Rheum. 2006. 54:387–389.
Article36. Junker JP, Sommar P, Skog M, Johnson H, Kratz G. Adipogenic, Chondrogenic and Osteogenic Differentiation of Clonally Derived Human Dermal Fibroblasts. Cells Tissues Organs. 2010. 07. 28. [Epub ahead of print].
Article37. Hoben GM, Willard VP, Athanasiou KA. Fibrochondrogenesis of hESCs: growth factor combinations and cocultures. Stem Cells Dev. 2009. 18:283–292.
Article38. Koay EJ, Athanasiou KA. Development of Serum-Free, Chemically Defined Conditions for Human Embryonic Stem Cell-Derived Fibrochondrogenesis. Tissue Eng Part A. 2009. 15:2249–2257.
Article39. Majumdar MK, Banks V, Peluso DP, Morris EA. Isolation, characterization, and chondrogenic potential of human bone marrow-derived multipotential stromal cells. J Cell Physiol. 2000. 185:98–106.
Article40. Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived Mesenchymal progenitor cells. Exp Cell Res. 1998. 238:265–272.
Article41. Elvenes J, Knutsen G, Johansen O, Moe BT, Martinez I. Development of a new method to harvest chondroprogenitor cells from underneath cartilage defects in the knees. J Orthop Sci. 2009. 14:410–417.
Article42. Grogan SP, Miyaki S, Asahara H, D'Lima DD, Lotz MK. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther. 2009. 11:R85.
Article43. Goessler UR, Bugert P, Bieback K, Stern-Straeter J, Bran G, Hömann K, Riedel F. Integrin expression in stem cells from bone marrow and adipose tissue during chondrogenic differentiation. Int J Mol Med. 2008. 21:271–279.
Article44. Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004. 50:1522–1532.
Article45. Koga H, Engebretsen L, Brinchmann JE, Muneta T, Sekiya I. Mesenchymal stem cell-based therapy for cartilage repair: a review. Knee Surg Sports Traumatol Arthrosc. 2009. 03. 31. [Epub ahead of print].
Article46. Van Osch GJ, Van Der Veen SW, Burger EH, Verwoerd-Verhoef HL. Chondrogenic potential of in vitro multiplied rabbit perichondrium cells cultured in alginate beads in defined medium. Tissue Eng. 2000. 6:321–330.
Article47. Wiesmann A, Buhring HJ, Mentrup C, Wiesmann HP. Decreased CD90 expression in human mesenchymal stem cells by applying mechanical stimulation. Head Face Med. 2006. 2:8.
Article48. Lee HJ, Chio BH, Min B-H, Park SR. Effects of low intensity ultrasound pretreatment on the chondrogenesis of rabbit mesenchymal stem cells. Tissue Eng Regener Med. 2005. 2:50–54.49. Diaz-Romero J, Gaillard JP, Grogan SP, Nesic D, Trub T, Mainil-Varlet P. Immunophenotypic analysis of human articular chondrocytes: changes in surface markers associated with cell expansion in monolayer culture. J Cell Physiol. 2005. 202:731–742.
Article50. Lee HJ, Choi BH, Min B-H, Park SR. Changes in surface markers of human mesenchymal stem cells during the chondrogenic differentiation and dedifferentiation processes in vitro. Arthritis & Rheumatism. 2009. 60:2325–2332.
Article51. Jin RL, Park SR, Choi BH, Min B-H. Scaffold-free cartilage fabrication system using passaged porcine chondrocytes and basic fibroblast growth factor. Tissue Eng Part A. 2009. 15:1887–1895.
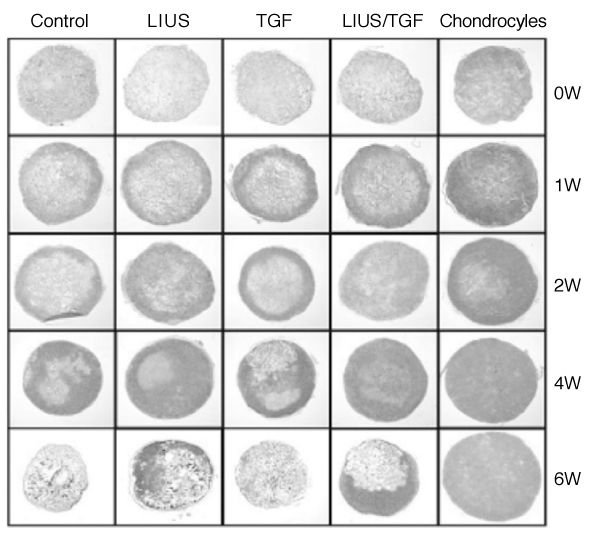
Article52. Cui JH, Park SR, Park K, Choi BH, Min B-H. Preconditioning of mesenchymal stem cells with low-intensity ultrasound for cartilage formation in vivo. Tissue Eng. 2007. 13:351–360.
Article53. Frenkel SR, Toolan B, Menche D, Pitman MI, Pachence JM. Chondrocyte transplantation using a collagen bilayer matrix for cartilage repair. J Bone Joint Surg Br. 1997. 79:831–836.
Article54. Marlovits S, Striessnig G, Kutscha-Lissberg F, Resinger C, Aldrian SM, Vécsei V, Trattnig S. Early postoperative adherence of matrix-induced autologous chondrocyte implantation for the treatment of full-thickness cartilage defects of the femoral condyle. Knee Surg Sports Traumatol Arthrosc. 2005. 13:451–457.
Article55. Funayama A, Niki Y, Matsumoto H, Maeno S, Yatabe T, Morioka H, Yanagimoto S, Taguchi T, Tanaka J, Toyama Y. Repair of full-thickness articular cartilage defects using injectable type II collagen gel embedded with cultured chondrocytes in a rabbit model. J Orthop Sci. 2008. 13:225–232.
Article56. Van Susante JL, Buma P, Schuman L, Homminga GN, van den Berg WB, Veth RP. Resurfacing potential of heterologous chondrocytes suspended in fibrin glue in large full-thickness defects of femoral articular cartilage: an experimental study in the goat. Biomaterials. 1999. 20:1167–1175.
Article57. Kim M, Shin Y, Hong B, Kim YJ, Chun JS, Tae G, Kim YH. In vitro chondrocyte culture in a heparin-based hydrogel for cartilage regeneration. Tissue Eng Part C Methods. 2009. 03. 27. [Epub ahead of print].58. Zheng L, Sun J, Chen X, Wang G, Jiang B, Fan H, Zhang X. In vivo cartilage engineering with collagen hydrogel and allogenous chondrocytes after diffusion chamber implantation in immunocompetent host. Tissue Eng Part A. 2009. 15:2145–2153.
Article59. Park S-H, Park SR, Chung SI, Pai KS, Min B-H. Tissue-engineered cartilage using fibrin/hyaluronan composite gel and its in vivo implantation. Artificial organs. 2005. 29:838–860.
Article60. Kawamura S, Wakitani S, Kimura T, Maeda A, Caplan AI, Shino K, Ochi T. Articular cartilage repair. Rabbit experiments with a collagen gel-biomatrix and chondrocytes cultured in it. Acta Orthop Scand. 1998. 69:56–62.
Article61. Homminga GN, Buma P, Koot HW, van der Kraan PM, van den Berg WB. Chondrocyte behavior in fibrin glue in vitro. Acta Orthop Scand. 1993. 64:441–445.
Article62. Diduch DR, Jordan LC, Mierisch CM, Balian G. Marrow stromal cells embedded in alginate for repair of osteochondral defects. Arthroscopy. 2000. 16:571–577.
Article63. Hoemann CD, Hurtig M, Rossomacha E, Sun J, Chevrier A, Shive MS, Buschmann MD. Chitosan-glycerol phos-phate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J Bone Joint Surg Am. 2005. 87:2671–2686.
Article64. Kim JK, Lee JS, Jung HJ, Cho JH, Heo JI, Chang YH. Preparation and properties of collagen/modified hyaluronic acid hydrogel for biomedical application. J Nanosci Nanotechnol. 2007. 7:3852–3856.
Article65. Marsich E, Borgogna M, Donati I, Mozetic P, Strand BL, Salvador SG, Vittur F, Paoletti S. Alginate/lactose-modified chitosan hydrogels: a bioactive biomaterial for chondrocyte encapsulation. J Biomed Mater Res A. 2008. 84:364–376.
Article66. Park S-H, Cui JH, Park SR, Min B-H. Potential of fortified fibrin/hyaluronic acid composite gel as a cell delivery vehicle for chondrocytes. Artificial organs. 2009. 33:439–447.
Article67. Kim HJ, Kim U-J, Vunjak-Novakovic G, Min B-H, Kaplan DL. Influence of macroporous protein scaffolds on bone tissue engineering from bone marrow stem cells. Biomaterials. 2005. 26:4442–4452.
Article68. Cui JH, Park K, Park SR, Min B-H. Effects of low-intensity ultrasound on chondrogenic differentiation of mesenchymal stem cells embedded in polyglycolic acid: an in vivo study. Tissue Eng. 2006. 12:75–82.
Article69. Lohmann CH, Schwartz Z, Niederauer GG, Carnes DL Jr, Dean DD, Boyan BD. Pretreatment with platelet derived growth factor-BB modulates the ability of costochondral resting zone chondrocytes incorporated into PLA/PGA scaffolds to form new cartilage in vivo. Biomaterials. 2000. 21:49–61.
Article70. Xin X, Hussain M, Mao JJ. Continuing differentiation of human mesenchymal stem cells and induced chondrogenic and osteogenic lineages in electrospun PLGA nanofiber scaffold. Biomaterials. 2007. 28:316–325.
Article71. Park K, Cho KJ, Kim JJ, Kim IH, Han DK. Functional PLGA scaffolds for chondrogenesis of bone-marrow-derived mesenchymal stem cells. Macromol Biosci. 2009. 9:221–229.
Article72. Jin CZ, Park SR, Choi BH, Park KD, Min B-H. In vivo cartilage tissue engineering using a cell-derived extracellular matrix scaffold. Artif Organs. 2007. 31:183–192.
Article73. Jin CZ, Choi BH, Park SR, Min B-H. Cartilage engineering using cell-derived extracellular matrix scaffold in vitro. J Biomed Mater Res A. 2009. 05. 12. [Epub ahead of print].74. Mobasheri A, Carter SD, Martín-Vasallo P, Shakibaei M. Integrins and stretch activated ion channels; putative components of functional cell surface mechanoreceptors in articular chondrocytes. Cell Biol Int. 2002. 26:1–18.
Article75. Williams GM, Klisch SM, Sah RL. Bioengineering cartilage growth, maturation, and form. Pediatr Res. 2008. 63(5):527–534.
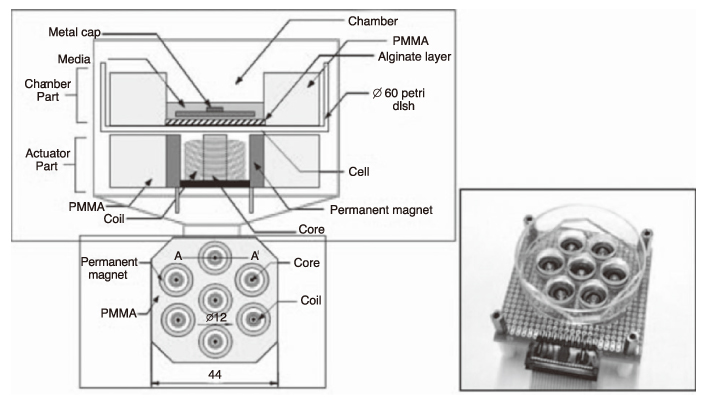
Article76. Park SR, Min B-H, Park S-H, Lee HJ. Application of mechanical stimulation for chondrogenesis. Tissue Eng Regen Med. 2005. 2:77–85.77. Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004. 22:80–86.
Article78. Angele P, Schumann D, Angele M, Kinner B, Englert C, Hente R, Fuchtmeier B, Nerlich M, Neumann C, Kujat R. Cyclic, mechanical compression enhances chondrogenesis of mesenchymal progenitor cells in tissue engineering scaffolds. Biorheology. 2004. 41:335–346.79. Angele P, Yoo JU, Smith C, Mansour J, Jepsen KJ, Nerlich M, Johnstone B. Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. J Orthop Res. 2003. 21:451–457.
Article80. Takahashi I, Nuckolls GH, Takahashi K, Tanaka O, Semba I, Dashner R, Shum L, Slavkin HC. Compressive force promotes SOX9, type II collagen and aggrecan and inhibits IL-1beta expression resulting in chondrogenesis in mouse embryonic limb bud mesenchymal cells. J Cell Sci. 1998. 111(Pt 14):2067–2076.
Article81. Park S-H, Sim WY, Park SW, Yang SS, Choi BH, Park SR, Park K, Min B-H. An electromagnetic compressive force by cell exciter stimulates chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. Tissue Eng. 2006. 12:3107–3117.
Article82. Sim WY, Park SW, Park S-H, Min B-H, Park SR, Yang SS. A pneumatic micro cell chip for the differentiation of human mesenchymal stem cells under mechanical stimulation. Lab chip. 2007. 7:1775–1782.
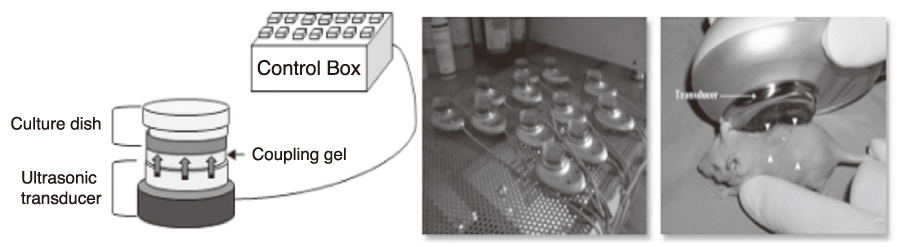
Article83. Park SR, Choi BH, Min B-H. Low-intensity ultrasound as an innovative tool for chondrogenesis of mesenchymal stem cells. Organogenesis. 2007. 3:74–78.
Article84. Mitragotri S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nature Reviews Drug Discovery. 2005. 4:255–260.
Article85. Choi BH, Woo J-I, Min B-H, Park SR. Low-intensity ultrasound stimulates the viability and matrix gene expression of human articular chondrocytes in alginate bead culture. J Biomed Mater Res A. 2006. 79:858–864.
Article86. Park SR, Park S-H, Jang KW, Cho HS, Cui JH, An HJ, Choi MJ, Chung SI, Min B-H. The effect of sonication on simulated osteoarthritis Part II: alleviation of osteoarthritis pathogenesis by 1 MHz ultrasound with simultaneous hyaluronate injection. Ultrasound Med Biol. 2005. 31:1559–1566.
Article87. Park SR, Jang KW, Park S-H, Cho HS, Jin CZ, Choi MJ, Chung SI, Min B-H. The effect of sonication on simulated osteoarthritis Part I: effects of 1 MHz ultrasound on uptake of hyaluronan into the rabbit synovium. Ultrasound Med Biol. 2005. 31:1551–1558.
Article88. Lee HJ, Choi BH, Min B-H, Park SR. Low-intensity ultrasound inhibits apoptosis and enhances viability of human mesenchymal stem cells in three-dimensional alginate culture during chondrogenic differentiation. Tissue Eng. 2007. 13:1049–1057.
Article89. Min B-H, Woo J-I, Cho H-S, Choi BH, Park S-J, Choi MJ, Park SR. Effects of low-intensity ultrasound stimulation of human cartilage explants. Scand J Rheumatol. 2006. 35:305–311.
Article90. Choi BH, Choi MH, Kwak MG, Min BH, Woo ZH, Park SR. Mechanotransduction pathways of low-intensity ultrasound in C-28/I2 human chondrocyte cell line. Proc Inst Mech Eng H. 2007. 221:527–535.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of Articular Cartilage Injury Using Mesenchymal Stem Cells
- Stimulation of Chondrogenic Differentiation of Mesenchymal Stem Cells
- Role of Mesenchymal Stem Cells in Patients with Nontraumatic Osteonecrosis of the Femoral Head
- Current Update of Cartilage Regeneration Using Stem Cells in Osteoarthritis
- Repair of Cartilage Defect by Cultured Mesenchymal Stem Cells from Bone Marrow