J Korean Orthop Assoc.
2007 Aug;42(4):537-544. 10.4055/jkoa.2007.42.4.537.
Synergistic Effect of Isoflavones on the BMP-4 MediatedOsteoblastic Differentiation of C2C12 Myoblast
- Affiliations
-
- 1Department of Orthopedic Surgery, College of Medicine, Chungbuk National University, Cheongju, Korea. ymkim@chungbuk.ac.kr
- 2Gyeonggi Regional Research Center, Hankyong National University, Anseong, Korea.
- 3Department of Biochemistry, College of Medicine, Chungbuk National University, Cheongju, Korea.
- KMID: 2186517
- DOI: http://doi.org/10.4055/jkoa.2007.42.4.537
Abstract
-
PURPOSE: Isoflavones are rich in soybean and are known to affect bone formation. This study examined the effects and modes of action of isoflavones on the differentiation of C2C12 myoblasts in the presence of the bone morphogenetic protein (BMP)-4.
MATERIALS AND METHODS
The isoflavones, daidzein, genistein or equol, and/or BMP-4 were added alone or in combination to C2C12 myoblasts. After 72 hours culture, the cells were stained for the early osteoblastic differentiation marker, alkaline phosphatase (ALP). The ALP activity was determined by comparing the color of the stained images as well as by spectrophotometry. The expression profiles of the extracellular matrix (ECM) genes responsible for the extensive remodeling at the cell surface were analyzed using agene expression microarray after treating thesamples with daidzein.
RESULTS
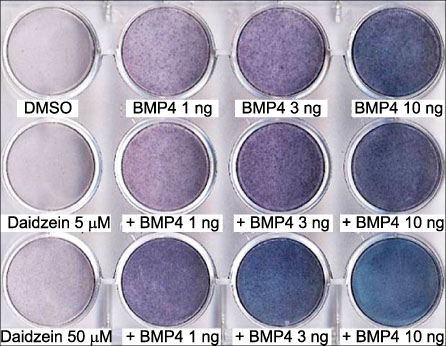
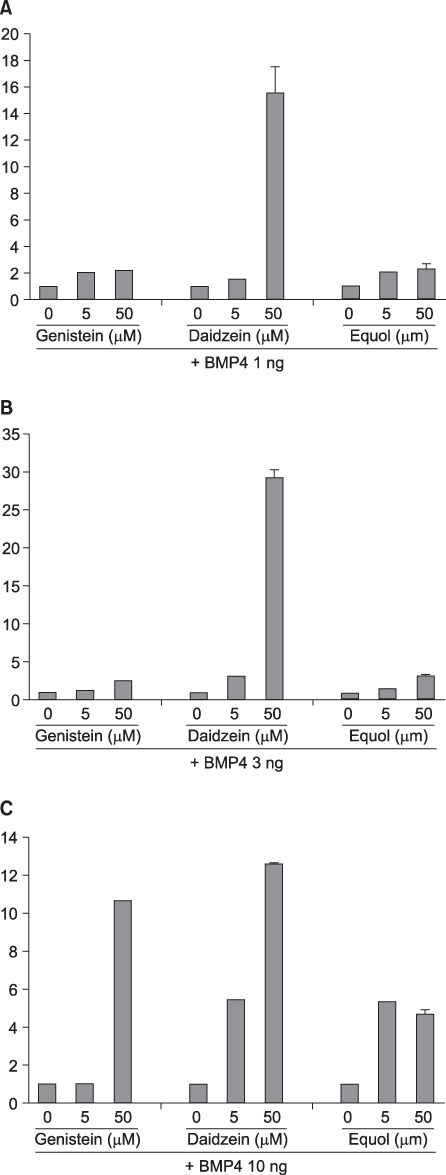
ALP staining of BMP-4 or the isoflavones-treated cells showed that BMP-4 increased the activity of ALP in a dose dependent manner, whereas the isoflavones alone did not induced any remarkable increase. However, the ALP activity increased when the cells were treated with BMP-4 and any of the three isoflavones. The macrogen mouse MAC array data showed that the ECM genes, Mmp13 and Mmp3, were up-regulated by daidzein, whereas Col4a2, Col5a1 and Mmp9 were down-regulated.
CONCLUSION
Isoflavones induce osteoblastic differentiation when combined with BMP-4, which is possibly achieved by modulating the expressional levels of various ECM genes.
Keyword
MeSH Terms
Figure
Reference
-
1. Anderson HC. Molecular biology of matrix vesicles. Clin Orthop Relat Res. 1995. 314:266–280.
Article2. Chandra A, Itakura T, Yang Z, et al. Neurogenesin-1 differentially inhibits the osteoblastic differentiation by bone morphogenetic proteins in C2C12 cells. Biochem Biophys Res Commun. 2006. 344:786–791.
Article3. Cheng H, Jiang W, Phillips FM, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am. 2003. 85:1544–1552.
Article4. Dang ZC, Lowik C. Dose-dependent effects of phytoestrogens on bone. Trends Endocrinol Metab. 2005. 16:207–213.
Article5. Fanti P, Monier-Faugere MC, Geng Z, et al. The phytoestrogen genistein reduces bone loss in short-term ovariectomized rats. Osteoporos Int. 1998. 8:274–281.
Article6. Gu Y, Zhu CF, Iwamoto H, Chen JS. Genistein inhibits invasive potential of human hepatocellular carcinoma by altering cell cycle, apoptosis, and angiogenesis. World J Gastroenterol. 2005. 11:6512–6517.
Article7. Inoue K, Mikuni-Takagaki Y, Oikawa K, et al. A crucial role for matrix metalloproteinase-2 in osteocytic canalicular formation and bone metabolism. J Biol Chem. 2006. 281:33814–33824.8. Ito C, Murata T, Itoigawa M, et al. Induction of apoptosis by isoflavonoids from the leaves of Millettia taiwaniana in human leukemia HL-60 cells. Planta Med. 2006. 72:424–429.9. Kanno S, Hirano S, Kayama F. Effects of phytoestrogens and environmental estrogens on osteoblastic differentiation in MC3T3-E1 cells. Toxicology. 2004. 196:137–145.
Article10. Katagiri T, Yamaguchi A, Komaki M, et al. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994. 127(6 Pt 1):1755–1766.11. Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998. 139:4252–4263.12. Kim H, Peterson TG, Barnes S. Mechanisms of action of the soy isoflavone genistein: emerging role for its effects via transforming growth factor beta signaling pathways. Am J Clin Nutr. 1998. 68:Suppl 6. S1418–S1425.
Article13. Klein BY, Gal I, Segal D. Studies of the levamisole inhibitory effect on rat stromal-cell commitment to mineralization. J Cell Biochem. 1993. 53:114–121.
Article14. Knight DC, Eden JA. A review of the clinical effects of phytoestrogens. Obstet Gynecol. 1996. 87(5 Pt 2):897–904.15. Krishnan V, Heath H, Bryant HU. Mechanism of action of estrogens and selective estrogen receptor modulators. Vitam Horm. 2000. 60:123–147.
Article16. Lee KS, Kim HJ, Li QL, et al. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol. 2000. 20:8783–8792.17. Malemud CJ. Matrix metalloproteinases: role in skeletal development and growth plate disorders. Front Biosci. 2006. 11:1702–1715.
Article18. Morabito N, Crisafulli A, Vergara C, et al. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: a randomized double-blind placebo-controlled study. J Bone Miner Res. 2002. 17:1904–1912.
Article19. Narisawa S, Hofmann MC, Ziomek CA, Millán JL. Embryonic alkaline phosphatase is expressed at M-phase in the spermatogenic lineage of the mouse. Development. 1992. 116:159–165.
Article20. Ripamonti U, Reddi AH. Growth and morphogenetic factors in bone induction: role of osteogenin and related bone morphogenetic proteins in craniofacial and periodontal bone repair. Crit Rev Oral Biol Med. 1992. 3:1–14.
Article21. Segev F, Héon E, Cole WG, et al. Structural abnormalities of the cornea and lid resulting from collagen V mutations. Invest Ophthalmol Vis Sci. 2006. 47:565–573.
Article22. Verma SP, Goldin BR. Effect of soy-derived isoflavonoids on the induced growth of MCF-7 cells by estrogenic environmental chemicals. Nutr Cancer. 1998. 30:232–239.
Article23. Wang CF, Kurzer MS. Effects of phytoestrogens on DNA synthesis in MCF-7 cells in the presence of estradiol or growth factors. Nutr Cancer. 1998. 31:90–100.
Article24. Wu HJ, Chan WH. Genistein protects methylglyoxal-induced oxidative DNA damage and cell injury in human mononuclear cells. Toxicol In Vitro. 2007. 21:335–342.
Article25. Xiao ZS, Hinson TK, Quarles LD. Cbfa1 isoform overexpression upregulates osteocalcin gene expression in non-osteoblastic and pre-osteoblastic cells. J Cell Biochem. 1999. 74:596–605.
Article26. Yamaguchi M, Sugimoto E. Stimulatory effect of genistein and daidzein on protein synthesis in osteoblastic MC3T3-E1 cells: activation of aminoacyl-tRNA synthetase. Mol Cell Biochem. 2000. 214:97–102.27. Zhu W, Rawlins BA, Boachie-Adjei O, et al. Combined bone morphogenetic protein-2 and -7 gene transfer enhances osteoblastic differentiation and spine fusion in a rodent model. J Bone Miner Res. 2004. 19:2021–2032.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nectandrin A Enhances the BMP-Induced Osteoblastic Differentiation and Mineralization by Activation of p38 MAPK-Smad Signaling Pathway
- A Possible Role of Kainate Receptors in C2C12 Skeletal Myogenic Cells
- Glucose Regulated Production of Human Insulin in Genetically Modified Myoblast Cell Line (C2C12)
- Comparative Study of BMP-2 Alone and Combined with VEGF Carried by Hydrogel for Maxillary Alveolar Bone Regeneration
- Effects of polygalacin D extracted from Platycodon grandiflorum on myoblast differentiation and muscle atrophy