J Breast Cancer.
2011 Mar;14(1):72-75. 10.4048/jbc.2011.14.1.72.
Microglandular Adenosis
- Affiliations
-
- 1Department of Surgery, Chungbuk National University, College of Medicine and Medical Research Institute, Cheongju, Korea. yjsong@chungbuk.ac.kr
- KMID: 2175647
- DOI: http://doi.org/10.4048/jbc.2011.14.1.72
Abstract
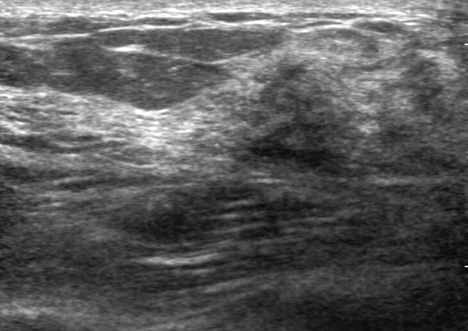
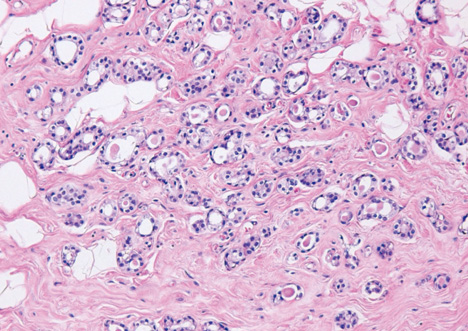
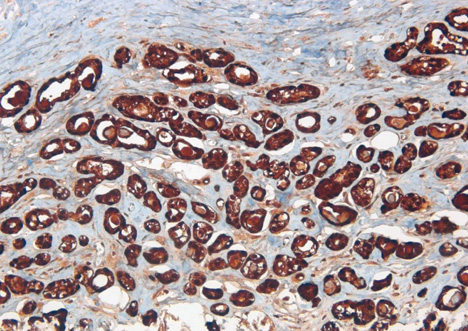
- Microglandular adenosis (MGA) of the breast is a very rare and benign proliferative lesion. Most patients complain of a palpable breast mass that may arouse a clinical suspicion of breast cancer. Histopathologically, it is hard to distinguish MGA from breast cancer because of the lack of a myoepithelial layer and infiltrative proliferation. Several studies have reported a strong relationship between MGA and carcinoma arising in MGA, so the mass should be excised completely in cases of MGA determined from a core needle biopsy rather than observation. A 72-years-old woman presented with a palpable breast mass. On physical examination, a mass was palpable in the right upper outer quadrant area and somewhat fixed to the surrounding tissues and pectoralis major muscle. We could not detect any mass or dense lesion on mammography because of a grade 4 dense breast. Ultrasonographic findings revealed a low echoic lesion with indistinct margins. The result of a core needle biopsy was MGA, which was confirmed by excision. We report one case of MGA, which was believed to breast cancer clinically.
Keyword
MeSH Terms
Figure
Reference
-
1. McDivitt RW, Stewart FW, Berg JW. Armed Forces Institute of Pathology (U.S.). Universities Associated for Research and Education in Pathology. Tumors of the breast. Atlas of Tumor Pathology, Second Serise, Fascicle 2. 1968. Wathington, D.C.: Armed Forces Institute of Pathology;91.2. Rosen PP. Rosen's Breast Pathology. 2009. 3rd ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins;175–186.3. Rosen PP. Microglandular adenosis. A benign lesion simulating invasive mammary carcinoma. Am J Surg Pathol. 1983. 7:137–144.4. Geyer FC, Kushner YB, Lambros MB, Natrajan R, Mackay A, Tamber N, et al. Microglandular adenosis or microglandular adenoma? A molecular genetic analysis of a case associated with atypia and invasive carcinoma. Histopathology. 2009. 55:732–743.
Article5. Eusebi V, Foschini MP, Betts CM, Gherardi G, Millis RR, Bussolati G, et al. Microglandular adenosis, apocrine adenosis, and tubular carcinoma of the breast. An immunohistochemical comparison. Am J Surg Pathol. 1993. 17:99–109.
Article6. Popper HH, Gallagher JV, Ralph G, Lenard PD, Tavassoli FA. Breast carcinoma arising in microglandular adenosis: a tumor expressing S-100 immunoreactivity. Report of five cases. Breast J. 1996. 2:154–159.
Article7. Tavassoli FA, Bratthauer GL. Immunohistochemical profile and differential diagnosis of microglandular adenosis. Mod Pathol. 1993. 6:318–322.8. James BA, Cranor ML, Rosen PP. Carcinoma of the breast arising in microglandular adenosis. Am J Clin Pathol. 1993. 100:507–513.
Article9. Shin SJ, Simpson PT, Da Silva L, Jayanthan J, Reid L, Lakhani SR, et al. Molecular evidence for progression of microglandular adenosis (MGA) to invasive carcinoma. Am J Surg Pathol. 2009. 33:496–504.
Article10. Diaz NM, McDivitt RW, Wick MR. Microglandular adenosis of the breast. An immunohistochemical comparison with tubular carcinoma. Arch Pathol Lab Med. 1991. 115:578–582.11. Koenig C, Dadmanesh F, Bratthauer GL, Tavassoli FA. Carcinoma arising in microglandular adenosis: an immunohistochemical analysis of 20 intraepithelial and invasive neoplasms. Int J Surg Pathol. 2000. 8:303–315.
Article12. Acs G, Simpson JF, Bleiweiss IJ, Hugh J, Reynolds C, Olson S, et al. Microglandular adenosis with transition into adenoid cystic carcinoma of the breast. Am J Surg Pathol. 2003. 27:1052–1060.
Article13. Khalifeh IM, Albarracin C, Diaz LK, Symmans FW, Edgerton ME, Hwang RF, et al. Clinical, histopathologic, and immunohistochemical features of microglandular adenosis and transition into in situ and invasive carcinoma. Am J Surg Pathol. 2008. 32:544–552.
Article14. Resetkova E, Flanders DJ, Rosen PP. Ten-year follow-up of mammary carcinoma arising in microglandular adenosis treated with breast conservation. Arch Pathol Lab Med. 2003. 127:77–80.
Article15. Salarieh A, Sneige N. Breast carcinoma arising in microglandular adenosis: a review of the literature. Arch Pathol Lab Med. 2007. 131:1397–1399.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ductal Carcinoma In Situ of the Breast Arising in Microglandular Adenosis
- Mammary Carcinoma Arising in Microglandular Adenosis: A Report of Five Cases
- Invasive Breast Carcinoma Arising in Microglandular Adenosis: Two Case Reports
- Metaplastic Carcinoma with Chondroid Differentiation Arising in Microglandular Adenosis
- Atypical form of Nodular Sclerosing Adenosis in the Breast: Mammography, US, and MR Imaging Findings: A Case Report