J Breast Cancer.
2013 Dec;16(4):432-437. 10.4048/jbc.2013.16.4.432.
Invasive Breast Carcinoma Arising in Microglandular Adenosis: Two Case Reports
- Affiliations
-
- 1Department of Surgery, Yeungnam University College of Medicine, Daegu, Korea.
- 2Department of Pathology, Yeungnam University College of Medicine, Daegu, Korea. ykbae@ynu.ac.kr
- KMID: 1976382
- DOI: http://doi.org/10.4048/jbc.2013.16.4.432
Abstract
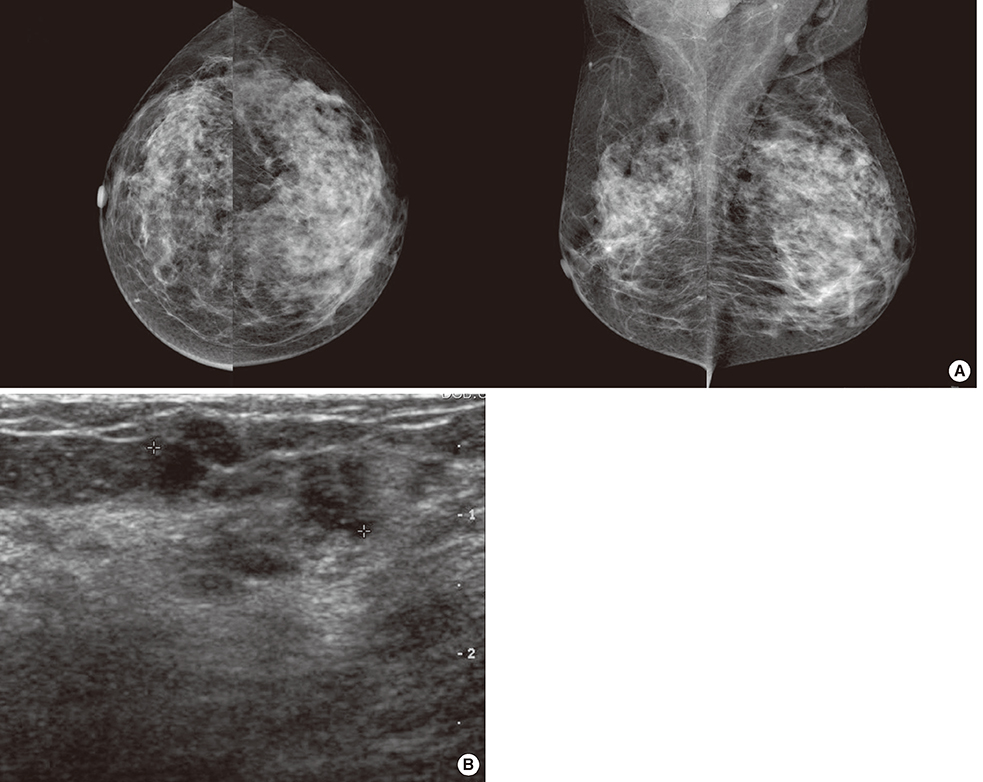
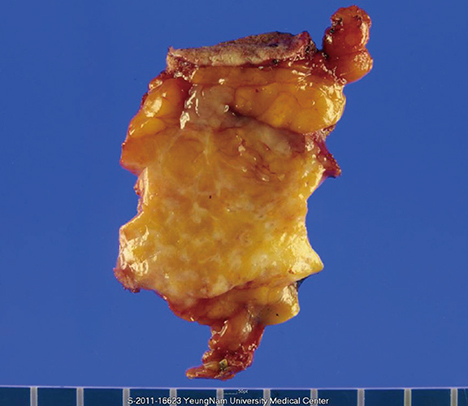
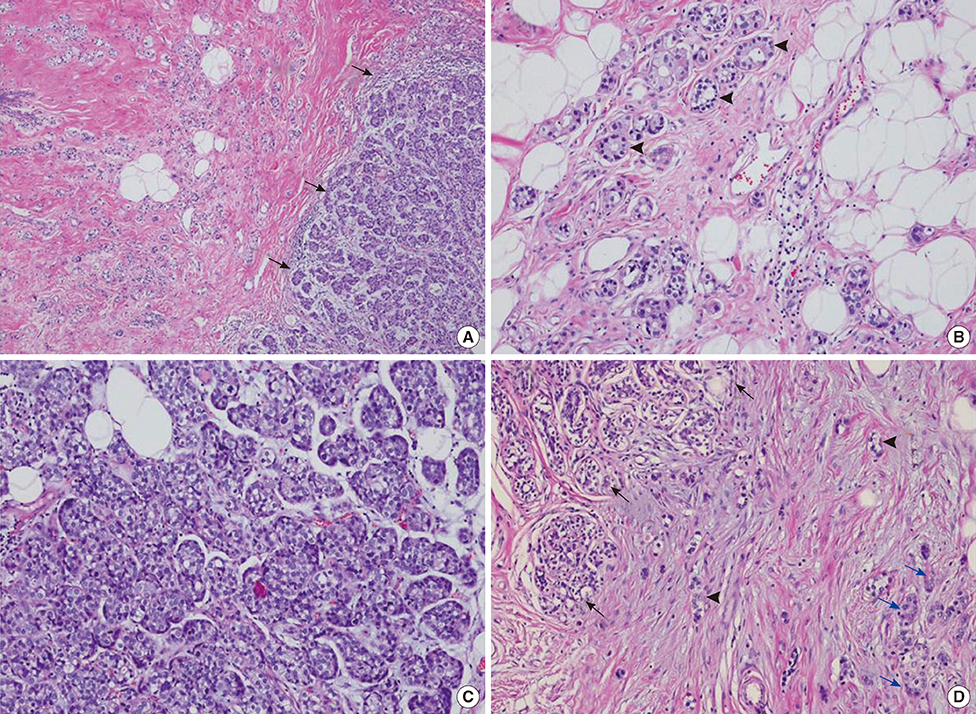
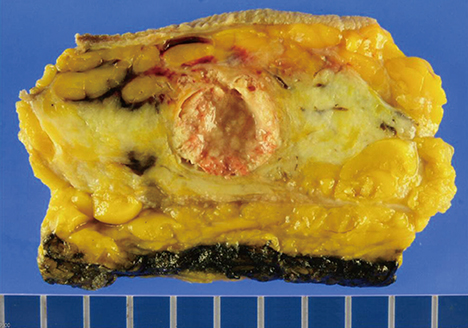
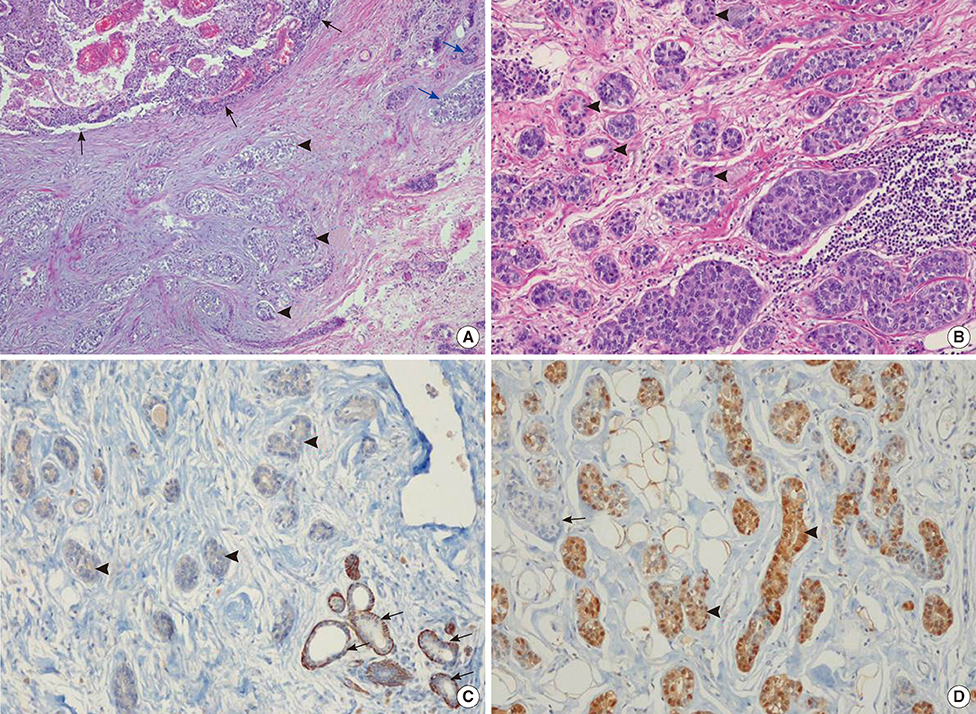
- Microglandular adenosis (MGA) is a rare benign disease that shows an infiltrative growth pattern of small glands, and it may progress to include atypia and carcinoma. Here we report two cases of breast carcinoma arising in MGA. Case 1 was a 44-year-old woman with a previous history of ductal carcinoma in situ in her right breast. During a follow-up, a 1.8 cm mass-like lesion was found in her left breast. An excisional biopsy suggested that the lesion was breast carcinoma. Case 2 was a 57-year-old woman with a 2.9 cm mass in her right breast. A core needle biopsy of the lesion suggested invasive carcinoma. Both patients underwent modified radical mastectomy with sentinel lymph node biopsy. Both tumors lacked a myoepithelial cell layer and stained positively for S-100, lysozyme, and alpha1-antitrypsin, which is typical of MGA. Both cases showed invasive carcinoma arising in MGA.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Metaplastic Carcinoma with Chondroid Differentiation Arising in Microglandular Adenosis
Ga-Eon Kim, Nah Ihm Kim, Ji Shin Lee, Min Ho Park
J Pathol Transl Med. 2017;51(4):418-421. doi: 10.4132/jptm.2016.10.06.
Reference
-
1. McDivitt RW, Stewart FW, Berg JW. Armed Forces Institute of Pathology (U.S.). Universities Associated for Research and Education in Pathology. Tumors of the breast. Atlas of Tumor Pathology, Second Serise, Fascicle 2. Wathington, DC: Armed Forces Institute of Pathology;1968. p. 91.2. Rosenblum MK, Purrazzella R, Rosen PP. Is microglandular adenosis a precancerous disease? A study of carcinoma arising therein. Am J Surg Pathol. 1986; 10:237–245.3. Resetkova E, Flanders DJ, Rosen PP. Ten-year follow-up of mammary carcinoma arising in microglandular adenosis treated with breast conservation. Arch Pathol Lab Med. 2003; 127:77–80.
Article4. Eusebi V, Foschini MP, Betts CM, Gherardi G, Millis RR, Bussolati G, et al. Microglandular adenosis, apocrine adenosis, and tubular carcinoma of the breast: an immunohistochemical comparison. Am J Surg Pathol. 1993; 17:99–109.
Article5. Koenig C, Dadmanesh F, Bratthauer GL, Tavassoli FA. Carcinoma arising in microglandular adenosis: an immunohistochemical analysis of 20 intraepithelial and invasive neoplasms. Int J Surg Pathol. 2000; 8:303–315.
Article6. James BA, Cranor ML, Rosen PP. Carcinoma of the breast arising in microglandular adenosis. Am J Clin Pathol. 1993; 100:507–513.
Article7. Tavassoli FA, Norris HJ. Microglandular adenosis of the breast: a clinicopathologic study of 11 cases with ultrastructural observations. Am J Surg Pathol. 1983; 7:731–737.8. Jeong MS, Kang JH, Kim EK, Woo JJ, Kim HS, An JK. Ductal carcinoma in situ of the breast arising in microglandular adenosis. J Korean Soc Radiol. 2012; 67:273–276.
Article9. Khalifeh IM, Albarracin C, Diaz LK, Symmans FW, Edgerton ME, Hwang RF, et al. Clinical, histopathologic, and immunohistochemical features of microglandular adenosis and transition into in situ and invasive carcinoma. Am J Surg Pathol. 2008; 32:544–552.
Article10. Damiani S, Pasquinelli G, Lamovec J, Peterse JL, Eusebi V. Acinic cell carcinoma of the breast: an immunohistochemical and ultrastructural study. Virchows Arch. 2000; 437:74–81.
Article11. Coyne JD, Dervan PA. Primary acinic cell carcinoma of the breast. J Clin Pathol. 2002; 55:545–547.
Article12. Schmitt FC, Ribeiro CA, Alvarenga S, Lopes JM. Primary acinic cell-like carcinoma of the breast: a variant with good prognosis? Histopathology. 2000; 36:286–289.
Article13. Rosen PP. Microglandular adenosis: a benign lesion simulating invasive mammary carcinoma. Am J Surg Pathol. 1983; 7:137–144.14. Rosen PP. Rosen's Breast Pathology. 3rd ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins;2008. p. 175–186.15. Salarieh A, Sneige N. Breast carcinoma arising in microglandular adenosis: a review of the literature. Arch Pathol Lab Med. 2007; 131:1397–1399.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ductal Carcinoma In Situ of the Breast Arising in Microglandular Adenosis
- Mammary Carcinoma Arising in Microglandular Adenosis: A Report of Five Cases
- Metaplastic Carcinoma with Chondroid Differentiation Arising in Microglandular Adenosis
- Microglandular Adenosis
- Lobular carcinoma in situ in sclerosing adenosis