Investig Magn Reson Imaging.
2015 Mar;19(1):1-9. 10.13104/imri.2015.19.1.1.
Reduced Gray Matter Volume of Auditory Cortical and Subcortical Areas in Congenitally Deaf Adolescents: A Voxel-Based Morphometric Study
- Affiliations
-
- 1Neuroimaging Lab., Neuroscience Research Institute, Kangwon National University Hospital, Chuncheon, Korea. wstae@kangwon.ac.kr
- KMID: 2175571
- DOI: http://doi.org/10.13104/imri.2015.19.1.1
Abstract
- PURPOSE
Several morphometric studies have been performed to investigate brain abnormalities in congenitally deaf people. But no report exists concerning structural brain abnormalities in congenitally deaf adolescents. We evaluated the regional volume changes in gray matter (GM) using voxel-based morphometry (VBM) in congenitally deaf adolescents.
MATERIALS AND METHODS
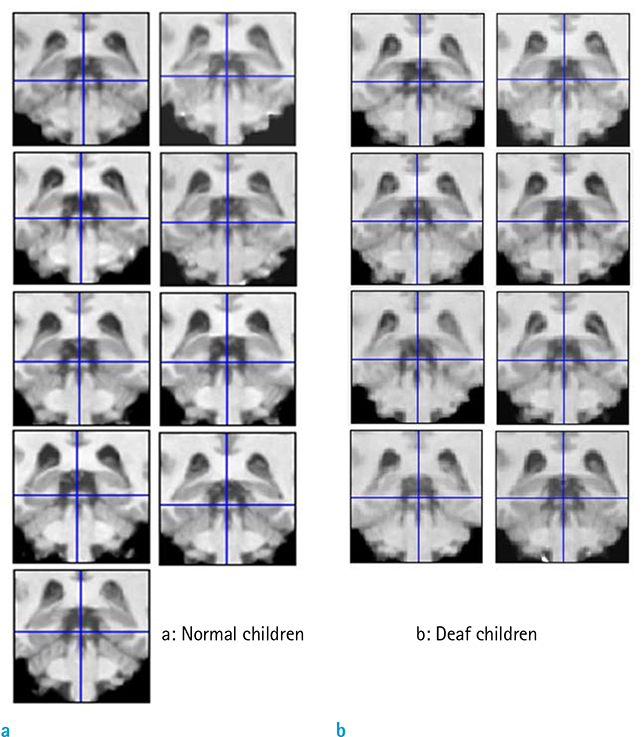
A VBM8 methodology was applied to the T1-weighted magnetic resonance imaging (MRI) scans of eight congenitally deaf adolescents (mean age, 15.6 years) and nine adolescents with normal hearing. All MRI scans were normalized to a template and then segmented, modulated, and smoothed. Smoothed GM data were tested statistically using analysis of covariance (controlled for age, gender, and intracranial cavity volume).
RESULTS
The mean values of age, gender, total volumes of GM, and total intracranial volume did not differ between the two groups. In the auditory centers, the left anterior Heschl's gyrus and both inferior colliculi showed decreased regional GM volume in the congenitally deaf adolescents. The GM volumes of the lingual gyri, nuclei accumbens, and left posterior thalamic reticular nucleus in the midbrain were also decreased.
CONCLUSIONS
The results of the present study suggest that early deprivation of auditory stimulation in congenitally deaf adolescents might have caused significant underdevelopment of the auditory cortex (left Heschl's gyrus), subcortical auditory structures (inferior colliculi), auditory gain controllers (nucleus accumbens and thalamic reticular nucleus), and multisensory integration areas (inferior colliculi and lingual gyri). These defects might be related to the absence of general auditory perception, the auditory gating system of thalamocortical transmission, and failure in the maturation of the auditory-to-limbic connection and the auditorysomatosensory-visual interconnection.
Keyword
MeSH Terms
Figure
Reference
-
1. Emmorey K, Allen JS, Bruss J, Schenker N, Damasio H. A morphometric analysis of auditory brain regions in congenitally deaf adults. Proc Natl Acad Sci U S A. 2003; 100:10049–10054.2. Kim DJ, Park SY, Kim J, Lee DH, Park HJ. Alterations of white matter diffusion anisotropy in early deafness. Neuroreport. 2009; 20:1032–1036.3. Penhune VB, Cismaru R, Dorsaint-Pierre R, Petitto LA, Zatorre RJ. The morphometry of auditory cortex in the congenitally deaf measured using MRI. Neuroimage. 2003; 20:1215–1225.4. Shibata DK. Differences in brain structure in deaf persons on MR imaging studied with voxel-based morphometry. AJNR Am J Neuroradiol. 2007; 28:243–249.5. Smith KM, Mecoli MD, Altaye M, et al. Morphometric differences in the Heschl's gyrus of hearing impaired and normal hearing infants. Cereb Cortex. 2011; 21:991–998.6. Morita T, Naito Y, Tsuji J, Nakamura T, Yamaguchi S, Ito J. Relationship between cochlear implant outcome and the diameter of the cochlear nerve depicted on MRI. Acta Otolaryngol Suppl. 2004; 56–59.7. Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001; 14:21–36.8. Douaud G, Gaura V, Ribeiro MJ, et al. Distribution of grey matter atrophy in Huntington's disease patients: a combined ROI-based and voxel-based morphometric study. Neuroimage. 2006; 32:1562–1575.9. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007; 38:95–113.10. Leonard CM, Puranik C, Kuldau JM, Lombardino LJ. Normal variation in the frequency and location of human auditory cortex landmarks Heschl's gyrus: where is it? Cereb Cortex. 1998; 8:397–406.11. Tahmasebi AM, Abolmaesumi P, Wild C, Johnsrude IS. A validation framework for probabilistic maps using Heschl's gyrus as a model. Neuroimage. 2010; 50:532–544.12. Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999; 7:254–266.13. Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapping. 1995; 2:189–210.14. Duvernoy HM. The human brain: surface, three-dimensional sectional anatomy and MRI. New York: Springer-Verlag;1999.15. Falchier A, Clavagnier S, Barone P, Kennedy H. Anatomical evidence of multimodal integration in primate striate cortex. J Neurosci. 2002; 22:5749–5759.16. Campbell R, MacSweeney M, Waters D. Sign language and the brain: a review. J Deaf Stud Deaf Educ. 2008; 13:3–20.17. Capek CM, Woll B, MacSweeney M, et al. Superior temporal activation as a function of linguistic knowledge: insights from deaf native signers who speechread. Brain Lang. 2010; 112:129–134.18. Finney EM, Fine I, Dobkins KR. Visual stimuli activate auditory cortex in the deaf. Nat Neurosci. 2001; 4:1171–1173.19. Neville HJ, Bavelier D, Corina D, et al. Cerebral organization for language in deaf and hearing subjects: biological constraints and effects of experience. Proc Natl Acad Sci U S A. 1998; 95:922–929.20. Moore JK, Guan YL. Cytoarchitectural and axonal maturation in human auditory cortex. J Assoc Res Otolaryngol. 2001; 2:297–311.21. Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004; 101:8174–8179.22. Abdul-Kareem IA, Sluming V. Heschl gyrus and its included primary auditory cortex: structural MRI studies in healthy and diseased subjects. J Magn Reson Imaging. 2008; 28:287–299.23. Giard MH, Peronnet F. Auditory-visual integration during multimodal object recognition in humans: a behavioral and electrophysiological study. J Cogn Neurosci. 1999; 11:473–490.24. Macaluso E, Frith CD, Driver J. Modulation of human visual cortex by crossmodal spatial attention. Science. 2000; 289:1206–1208.25. Schmithorst VJ, Holland SK. The effect of musical training on the neural correlates of math processing: a functional magnetic resonance imaging study in humans. Neurosci Lett. 2004; 354:193–196.26. Bavelier D, Neville HJ. Cross-modal plasticity: where and how? Nat Rev Neurosci. 2002; 3:443–452.27. Shore SE. Auditory/somatosensory interactions. In : Squire LR, editor. Encyclopedia of neuroscience. San Diego: Academic Press;2009. p. 691–695.28. Sokoloff L, Reivich M, Kennedy C, et al. The [14C] deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977; 28:897–916.29. Blood AJ, Zatorre RJ, Bermudez P, Evans AC. Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nat Neurosci. 1999; 2:382–387.30. Zald DH, Pardo JV. The neural correlates of aversive auditory stimulation. Neuroimage. 2002; 16:746–753.31. Muhlau M, Rauschecker JP, Oestreicher E, et al. Structural brain changes in tinnitus. Cereb Cortex. 2006; 16:1283–1288.32. McCullough LD, Sokolowski JD, Salamone JD. A neurochemical and behavioral investigation of the involvement of nucleus accumbens dopamine in instrumental avoidance. Neuroscience. 1993; 52:919–925.33. Jones EG. The thalamus. 2nd ed. Cambridge, UK: Cambridge University Press;2007.34. Rauschecker JP, Leaver AM, Muhlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010; 66:819–826.35. O'Donnell P, Lavin A, Enquist LW, Grace AA, Card JP. Interconnected parallel circuits between rat nucleus accumbens and thalamus revealed by retrograde transynaptic transport of pseudorabies virus. J Neurosci. 1997; 17:2143–2167.36. Yu XJ, Xu XX, Chen X, He S, He J. Slow recovery from excitation of thalamic reticular nucleus neurons. J Neurophysiol. 2009; 101:980–987.37. Kim JH, Kim SH, Shin HS, et al. A study of changes of inversion time effect on brain volume of normal volunteers. J Korean Soc Magn Reson Med. 2013; 17:286–293.38. Jung WB, Kang MJ, Son DB, et al. Reproducibility analysis of brain volumetry measured from inter MR scanner of multi-institute. J Korean Soc Magn Reson Med. 2012; 16:243–252.39. Jung WB, Son DB, Kim YJ, Kim YH, Eun CK, Mun CW. A comparison study on human brain volume of white matter, gray matter and hippocampus depending on magnetic resonance imaging conditions and applied brain template. J Korean Soc Magn Reson Med. 2011; 15:242–250.40. Choi N, Nam Y, Kim DH. Cortical thickness estimation using DIR imaging with GRAPPA factor 2. J Korean Soc Magn Reson Med. 2010; 14:56–63.41. Kim HD, Chang KH, Han MH, Kim HJ, Lee SG, Lee MC. The significance and limitation of MR volumetry: comparison between normal adults and the patients with epilepsy and hippocampal sclerosis. J Korean Soc Magn Reson Med. 2002; 6:47–54.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Voxel Based Morphometric Analysis of Longitudinal Cortical Gray Matter Changes in Progranulin Mutation Carriers At-Risk for Frontotemporal Dementia: Preliminary Study
- Reduced Gray Matter Volume in Subjective Cognitive Decline: A Voxel-Based Morphometric Study
- Voxel-based Morphometry (VBM) Based Assessment of Gray Matter Loss in Medial Temporal Lobe Epilepsy: Comparison with FDG PET
- Gray Matter Heterotopias: MR and Clinical Features
- A Voxel-Based Morphometry of Gray Matter Reduction in Patients with Dementia of the Alzheimer's Type