Diabetes Metab J.
2013 Jun;37(3):181-189. 10.4093/dmj.2013.37.3.181.
Safety and Efficacy of Modern Insulin Analogues
- Affiliations
-
- 1Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea. 103hyun@korea.ac.kr
- 2Department of Internal Medicine, Konyang University College of Medicine, Daejeon, Korea.
- 3Department of Internal Medicine, Eulji University School of Medicine, Daejeon, Korea.
- 4Department of Internal Medicine, Kyung Hee University School of Medicine, Seoul, Korea.
- 5Department of Internal Medicine, Eulji University School of Medicine, Seoul, Korea.
- 6Department of Internal Medicine, Inje University College of Medicine, Busan, Korea.
- 7Department of Internal Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 8Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 9Department of Internal Medicine, Inha University School of Medicine, Incheon, Korea.
- 10Department of Internal Medicine, Chungbuk National University College of Medicine, Cheongju, Korea.
- 11Department of Internal Medicine, University of Ulsan College of Medicine, Ulsan, Korea.
- 12Department of Internal Medicine, Hongik Hospital, Seoul, Korea.
- 13Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea.
- 14Department of Internal Medicine, Kosin University College of Medicine, Busan, Korea.
- 15Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea.
- 16Novo Nordisk Pharma Korea, Seoul, Korea.
- KMID: 2174292
- DOI: http://doi.org/10.4093/dmj.2013.37.3.181
Abstract
- BACKGROUND
A1chieve(R) was a noninterventional study evaluating the clinical safety and efficacy of biphasic insulin aspart 30, insulin detemir, and insulin aspart.
METHODS
Korean type 2 diabetes patients who have not been treated with the study insulin or have started it within 4 weeks before enrollment were eligible for the study. The patient selection and the choice of regimen were at the discretion of the physician. The safety and efficacy information was collected from the subjects at baseline, week 12, and week 24. The number of serious adverse drug reactions (SADRs) was the primary endpoint. The changes of clinical diabetic markers at week 12 and/or at week 24 compared to baseline were the secondary endpoints.
RESULTS
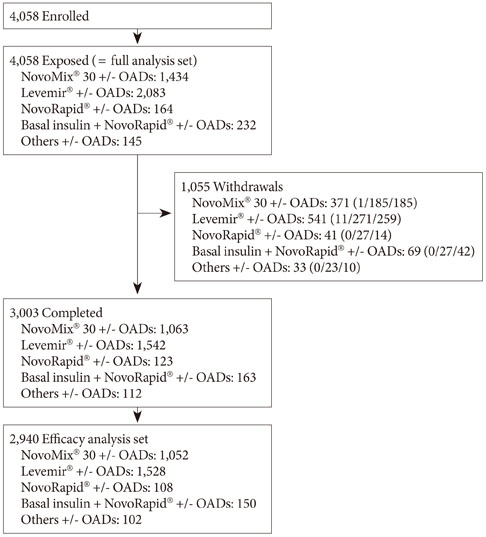
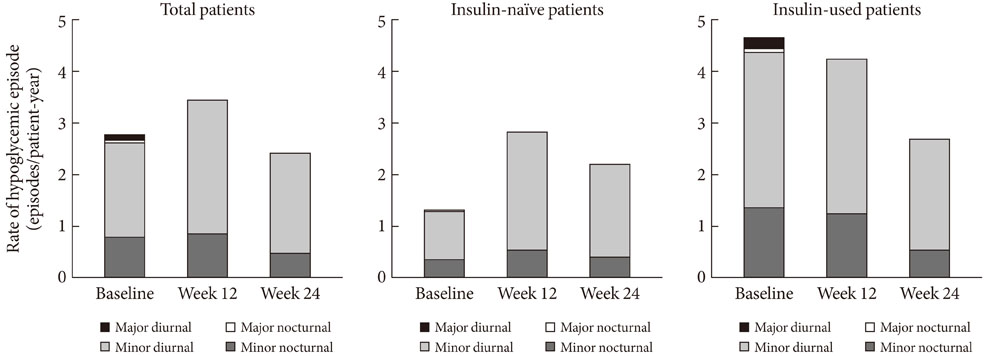
Out of 4,058 exposed patients, 3,003 completed the study. During the study period, three SADRs were reported in three patients (0.1%). No major hypoglycemic episodes were observed and the rate of minor hypoglycemic episodes marginally decreased during 24 weeks (from 2.77 to 2.42 events per patient-year). The overall quality of life score improved (from 66.7+/-15.9 to 72.5+/-13.5) while the mean body weight was slightly increased (0.6+/-3.0 kg). The 24-week reductions in glycated hemoglobin, fasting plasma glucose and postprandial plasma glucose were 1.6%+/-2.2%, 2.5+/-4.7 mmol/L, and 4.0+/-6.4 mmol/L, respectively.
CONCLUSION
The studied regimens showed improvements in glycemic control with low incidence of SADRs, including no incidence of major hypoglycemic episodes in Korean patients with type 2 diabetes.
MeSH Terms
-
Biphasic Insulins
Body Weight
Diabetes Mellitus, Type 2
Drug Toxicity
Fasting
Glucose
Hemoglobins
Humans
Incidence
Insulin
Insulin Aspart
Insulin, Isophane
Insulin, Long-Acting
Patient Selection
Plasma
Quality of Life
Republic of Korea
Treatment Outcome
Insulin Detemir
Biphasic Insulins
Glucose
Hemoglobins
Insulin
Insulin Aspart
Insulin, Isophane
Insulin, Long-Acting
Figure
Cited by 1 articles
-
Characteristics Predictive for a Successful Switch from Insulin Analogue Therapy to Oral Hypoglycemic Agents in Patients with Type 2 Diabetes
Gyuri Kim, Yong-ho Lee, Eun Seok Kang, Bong-Soo Cha, Hyun Chul Lee, Byung-Wan Lee
Yonsei Med J. 2016;57(6):1395-1403. doi: 10.3349/ymj.2016.57.6.1395.
Reference
-
1. Gaster B, Hirsch IB. The effects of improved glycemic control on complications in type 2 diabetes. Arch Intern Med. 1998; 158:134–140.2. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000; 321:405–412.3. Saaddine JB, Cadwell B, Gregg EW, Engelgau MM, Vinicor F, Imperatore G, Narayan KM. Improvements in diabetes processes of care and intermediate outcomes: United States, 1988-2002. Ann Intern Med. 2006; 144:465–474.4. Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004; 291:335–342.5. Riddle MC. Timely initiation of basal insulin. Am J Med. 2004; 116:Suppl 3A. 3S–9S.6. Wright A, Burden AC, Paisey RB, Cull CA, Holman RR; U.K. Prospective Diabetes Study Group. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. Prospective Diabetes Study (UKPDS 57). Diabetes Care. 2002; 25:330–336.7. Korytkowski M. When oral agents fail: practical barriers to starting insulin. Int J Obes Relat Metab Disord. 2002; 26:Suppl 3. S18–S24.8. McCrimmon RJ, Frier BM. Hypoglycaemia, the most feared complication of insulin therapy. Diabete Metab. 1994; 20:503–512.9. Peyrot M, Rubin RR, Lauritzen T, Skovlund SE, Snoek FJ, Matthews DR, Landgraf R, Kleinebreil L. International DAWN Advisory Panel. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005; 28:2673–2679.10. Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care. 2006; 29:1269–1274.11. Philis-Tsimikas A, Charpentier G, Clauson P, Ravn GM, Roberts VL, Thorsteinsson B. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther. 2006; 28:1569–1581.12. Dornhorst A, Luddeke HJ, Koenen C, Merilainen M, King A, Robinson A, Sreenan S. PREDICTIVE Study Group. Transferring to insulin detemir from NPH insulin or insulin glargine in type 2 diabetes patients on basal-only therapy with oral antidiabetic drugs improves glycaemic control and reduces weight gain and risk of hypoglycaemia: 14-week follow-up data from PREDICTIVE. Diabetes Obes Metab. 2008; 10:75–81.13. Fajardo Montanana C, Hernandez Herrero C, Rivas Fernandez M. Less weight gain and hypoglycaemia with once-daily insulin detemir than NPH insulin in intensification of insulin therapy in overweight type 2 diabetes patients: the PREDICTIVE BMI clinical trial. Diabet Med. 2008; 25:916–923.14. Sharma SK, Al-Mustafa M, Oh SJ, Azar ST, Shestakova M, Guler S, Vaz JA. Biphasic insulin aspart 30 treatment in patients with type 2 diabetes poorly controlled on prior diabetes treatment: results from the PRESENT study. Curr Med Res Opin. 2008; 24:645–652.15. Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. American Diabetes Association. European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009; 32:193–203.16. Donner T, Munoz M. Update on insulin therapy for type 2 diabetes. J Clin Endocrinol Metab. 2012; 97:1405–1413.17. Bonds DE, Miller ME, Bergenstal RM, Buse JB, Byington RP, Cutler JA, Dudl RJ, Ismail-Beigi F, Kimel AR, Hoogwerf B, Horowitz KR, Savage PJ, Seaquist ER, Simmons DL, Sivitz WI, Speril-Hillen JM, Sweeney ME. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010; 340:b4909.18. Evans M, Schumm-Draeger PM, Vora J, King AB. A review of modern insulin analogue pharmacokinetic and pharmacodynamic profiles in type 2 diabetes: improvements and limitations. Diabetes Obes Metab. 2011; 13:677–684.19. Shah S, Benroubi M, Borzi V, Gumprecht J, Kawamori R, Shaban J, Shestakova M, Wenying Y, Valensi P. IMPROVE Study Group Expert Panel. Safety and effectiveness of biphasic insulin aspart 30/70 (NovoMix 30) when switching from human premix insulin in patients with type 2 diabetes: subgroup analysis from the 6-month IMPROVE observational study. Int J Clin Pract. 2009; 63:574–582.20. Raskin P, Gylvin T, Weng W, Chaykin L. Comparison of insulin detemir and insulin glargine using a basal-bolus regimen in a randomized, controlled clinical study in patients with type 2 diabetes. Diabetes Metab Res Rev. 2009; 25:542–548.21. Hallschmid M, Jauch-Chara K, Korn O, Molle M, Rasch B, Born J, Schultes B, Kern W. Euglycemic infusion of insulin detemir compared with human insulin appears to increase direct current brain potential response and reduces food intake while inducing similar systemic effects. Diabetes. 2010; 59:1101–1107.22. Hendriksen KV, Jensen T, Oturai P, Feldt-Rasmussen B. Effects of insulin detemir and NPH insulin on renal handling of sodium, fluid retention and weight in type 2 diabetic patients. Diabetologia. 2012; 55:46–50.23. Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes Metab Res Rev. 1999; 15:205–218.24. van der Does FE, de Neeling JN, Snoek FJ, Grootenhuis PA, Kostense PJ, Bouter LM, Heine RJ. Randomized study of two different target levels of glycemic control within the acceptable range in type 2 diabetes. Effects on well-being at 1 year. Diabetes Care. 1998; 21:2085–2093.25. Home P, Naggar NE, Khamseh M, Gonzalez-Galvez G, Shen C, Chakkarwar P, Wenying Y. An observational non-interventional study of people with diabetes beginning or changed to insulin analogue therapy in non-Western countries: the A1chieve study. Diabetes Res Clin Pract. 2011; 94:352–363.26. Yang W, Zilov A, Soewondo P, Bech OM, Sekkal F, Home PD. Observational studies: going beyond the boundaries of randomized controlled trials. Diabetes Res Clin Pract. 2010; 88:Suppl 1. S3–S9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Unusually Elevated Serum Insulin Level in a Diabetic Patient during Recombinant Insulin Therapy
- The Effects of Anti-insulin Antibodies and Cross-reactivity with Human Recombinant Insulin Analogues in the E170 Insulin Immunometric Assay
- Sulfonylurea, an Update
- Clinical Utility and Cross-Reactivity of Insulin and C-Peptide Assays by the Lumipulse G1200 System
- Clinical Efficacy of Glucagon Like Peptide-1 (GLP-1) Analogues