Korean J Lab Med.
2011 Jan;31(1):22-29. 10.3343/kjlm.2011.31.1.22.
The Effects of Anti-insulin Antibodies and Cross-reactivity with Human Recombinant Insulin Analogues in the E170 Insulin Immunometric Assay
- Affiliations
-
- 1Department of Laboratory Medicine, Konkuk University School of Medicine, Seoul, Korea. ymyun@kuh.ac.kr
- 2Institute of Biomedical Science and Technology, Konkuk University, Seoul, Korea.
- KMID: 1096656
- DOI: http://doi.org/10.3343/kjlm.2011.31.1.22
Abstract
- BACKGROUND
Insulin assays are affected by varying degrees of interference from anti-insulin antibodies (IAs) and by cross-reactivity with recombinant insulin analogues. We evaluated the usefulness of the E170 insulin assay by assessing IA effects and cross-reactivity with 2 analogues.
METHODS
Sera were obtained from 59 type 2 diabetes patients receiving continuous subcutaneous insulin infusion and 18 healthy controls. Insulin levels were determined using an E170 analyzer. To investigate the effects of IAs, we performed IA radioimmunoassays, and analyzed the differences between directly measured insulin (direct insulin) and polyethylene glycol (PEG)-treated insulins (free, IA-unbound; total, IA-bound and unbound insulin). We performed in-vitro cross-reactivity tests with insulin aspart and insulin glulisine.
RESULTS
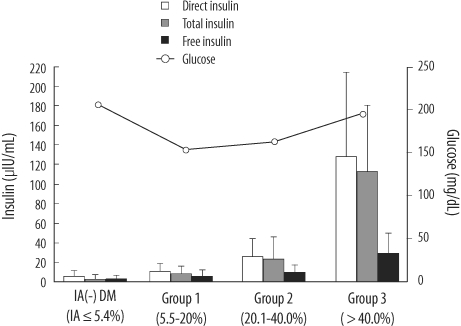
In IA-positive patients, E170 free insulin levels measured using the E170 analyzer were significantly lower than the direct insulin levels. The mean value of the direct/free insulin ratio and IA-bound insulin, which were calculated as the difference between total and free insulin, increased significantly as endogenous IA levels increased. The E170 insulin assay showed low cross-reactivities with both analogues (< 0.7%).
CONCLUSIONS
IAs interfered with E170 insulin assay, and the extent of interference correlated with the IA levels, which may be attributable to the increase in IA-bound insulin, and not to an error in the assay. The E170 insulin assay may measure only endogenous insulin since cross-reactivity is low. Our results suggest that the measurement of free insulin after PEG pre-treatment could be useful for beta cell function assessment in diabetic patients undergoing insulin therapy.
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Cross Reactions
Diabetes Mellitus, Type 2/blood/immunology
Female
Humans
Infusions, Subcutaneous
Insulin/analogs & derivatives/*blood/chemistry/immunology
Insulin Antibodies/*blood
Male
Middle Aged
Polyethylene Glycols/chemistry
Radioimmunoassay/instrumentation/*methods
Recombinant Proteins/analysis/immunology/metabolism
Figure
Cited by 1 articles
-
Unusually Elevated Serum Insulin Level in a Diabetic Patient during Recombinant Insulin Therapy
Serim Kim, Yeo-Min Yun, Mina Hur, Hee-Won Moon
Lab Med Online. 2013;3(1):56-59. doi: 10.3343/lmo.2013.3.1.56.
Reference
-
1. Sapin R. Insulin immunoassays: fast approaching 50 years of existence and still calling for standardization. Clin Chem. 2007; 53:810–812. PMID: 17468407.
Article2. Radermecker RP, Renard E, Scheen AJ. Circulating insulin antibodies: influence of continuous subcutaneous or intraperitoneal insulin infusion, and impact on glucose control. Diabetes Metab Res Rev. 2009; 25:491–501. PMID: 19496088.
Article3. Sapin R. Insulin assays: previously known and new analytical features. Clin Lab. 2003; 49:113–121. PMID: 12705692.4. Walker JM, editor. The protein protocols handbook. 2002. 2nd ed. New Jersey: Humana Press;p. 991–992.5. Kuzuya H, Blix PM, Horwitz DL, Steiner DF, Rubenstein AH. Determination of free and total insulin and C-peptide in insulin-treated diabetics. Diabetes. 1977; 26:22–29. PMID: 830562.
Article6. Gennaro WD, Van Norman JD. Quantitation of free, total, and antibody-bound insulin in insulin-treated diabetics. Clin Chem. 1975; 21:873–879. PMID: 236845.
Article7. Armitage M, Wilkin T, Wood P, Casey C, Loveless R. Insulin autoantibodies and insulin assay. Diabetes. 1988; 37:1392–1396. PMID: 3046970.
Article8. Sapin R. Anti-insulin antibodies in insulin immunometric assays: a still possible pitfall. Eur J Clin Chem Clin Biochem. 1997; 35:365–367. PMID: 9189740.
Article9. Sapin R. The interference of insulin antibodies in insulin immunometric assays. Clin Chem Lab Med. 2002; 40:705–708. PMID: 12241018.
Article10. Hanning I, Home PD, Alberti KG. Measurement of free insulin concentrations: the influence of the timing of extraction of insulin antibodies. Diabetologia. 1985; 28:831–835. PMID: 3910494.
Article11. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993; 329:977–986. PMID: 8366922.12. Jeandidier N, Boivin S, Sapin R, Rosart-Ortega F, Uring-Lambert B, Réville P, et al. Immunogenicity of intraperitoneal insulin infusion using programmable implantable devices. Diabetologia. 1995; 38:577–584. PMID: 7489841.
Article13. Fineberg SE, Kawabata TT, Finco-Kent D, Fountaine RJ, Finch GL, Krasner AS. Immunological responses to exogenous insulin. Endocr Rev. 2007; 28:625–652. PMID: 17785428.
Article14. Owen WE, Roberts WL. Cross-reactivity of three recombinant insulin analogs with five commercial insulin immunoassays. Clin Chem. 2004; 50:257–259. PMID: 14709671.
Article15. Ibrahim F, Bagnard G, Boudou P. Differences in circulating insulin levels following glargine administration. Clin Biochem. 2008; 41:429–431. PMID: 18160047.
Article16. Becker RH. Insulin glulisine complementing basal insulins: a review of structure and activity. Diabetes Technol Ther. 2007; 9:109–121. PMID: 17316105.17. De Meyts P. Insulin and its receptor: structure, function and evolution. Bioessays. 2004; 26:1351–1362. PMID: 15551269.
Article18. Devendra D, Galloway TS, Horton SJ, Evenden A, Keller U, Wilkin TJ. The use of phage display to distinguish insulin autoantibody (IAA) from insulin antibody (IA) idiotypes. Diabetologia. 2003; 46:802–809. PMID: 12783163.
Article19. Kim HR, Lee MK, Park AJ. The -308 and -238 polymorphisms of the TNF- promoter gene in type 2 diabetes mellitus. Korean J Lab Med. 2006; 26:58–63. PMID: 18156701.
Article20. Berson SA, Yalow RS. Quantitative aspects of the reaction between insulin and insulin-binding antibody. J Clin Invest. 1959; 38:1996–2016. PMID: 13799922.
Article21. Kahn CR, Rosenthal AS. Immunologic reactions to insulin: insulin allergy, insulin resistance, and the autoimmune insulin syndrome. Diabetes Care. 1979; 2:283–295. PMID: 510122.
Article22. Grunfeld C. Insulin resistance: pathophysiology, diagnosis, and therapeutic implications. Spec Top Endocrinol Metab. 1984; 6:193–240. PMID: 6395415.23. Davidson JK, DeBra DW. Immunologic insulin resistance. Diabetes. 1978; 27:307–318. PMID: 640236.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Unusually Elevated Serum Insulin Level in a Diabetic Patient during Recombinant Insulin Therapy
- Clinical Utility and Cross-Reactivity of Insulin and C-Peptide Assays by the Lumipulse G1200 System
- Spontaneous Hypoglycemia due to Insulin Antibody after Insulin Treatment of Diabetic Ketoacidosis
- Purification of human RBC insulin receptor by high performance insulin affinity column
- Two Cases of hypoglycemia in IDDM patients with insulin antibody