Diabetes Metab J.
2013 Dec;37(6):465-474. 10.4093/dmj.2013.37.6.465.
Glycemic Effectiveness of Metformin-Based Dual-Combination Therapies with Sulphonylurea, Pioglitazone, or DPP4-Inhibitor in Drug-Naive Korean Type 2 Diabetic Patients
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. bwanlee@yuhs.ac
- 2Department of Medicine, The Graduate School of Yonsei University, Seoul, Korea.
- 3Division of Endocrinology, Department of Internal Medicine, National Health Insurance Corporation Ilsan Hospital, Goyang, Korea.
- KMID: 2174228
- DOI: http://doi.org/10.4093/dmj.2013.37.6.465
Abstract
- BACKGROUND
This study compared the glycemic effectiveness of three metformin-based dual therapies according to baseline hemoglobin A1c (HbA1c) to evaluate the appropriateness of the guideline enforced by the National Health Insurance Corporation of Korea for initial medication of type 2 diabetes (T2D).
METHODS
This prospective observational study was conducted across 24 weeks for drug-naive Korean T2D patients with HbA1c greater than 7.5%. Subjects were first divided into three groups based on the agent combined with metformin (group 1, gliclazide-modified release or glimepiride; group 2, pioglitazone; group 3, sitagliptin). Subjects were also classified into three categories according to baseline HbA1c (category I, 7.5%< or =HbA1c<9.0%; category II, 9.0%< or =HbA1c<11.0%; category III, 11.0%< or =HbA1c).
RESULTS
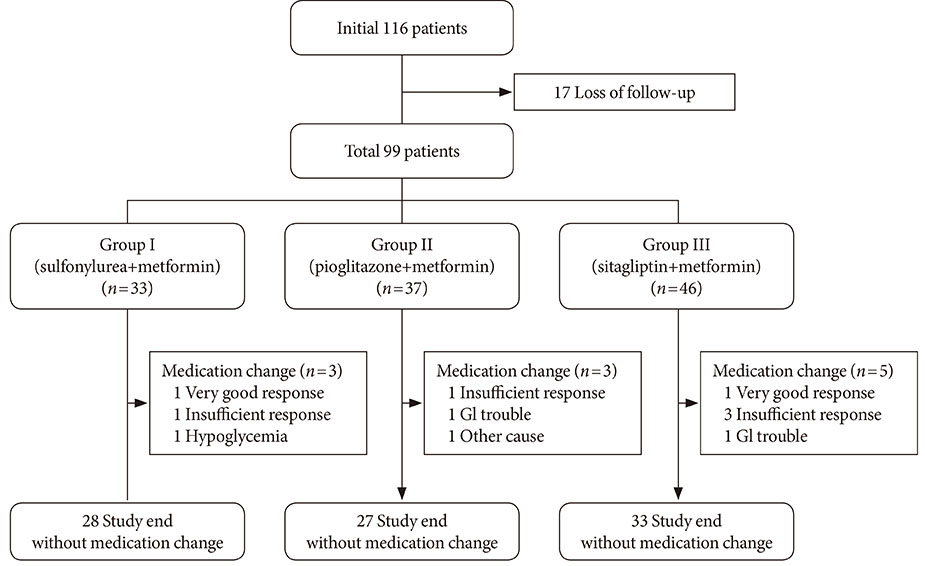
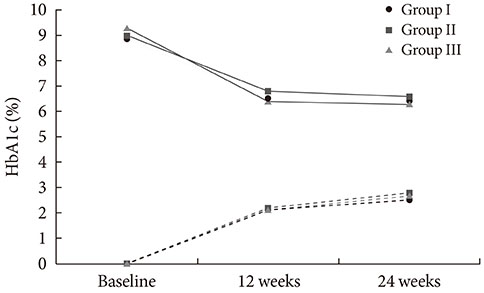
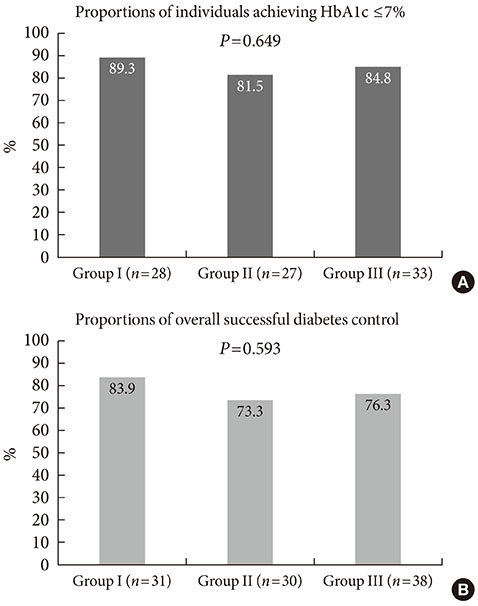
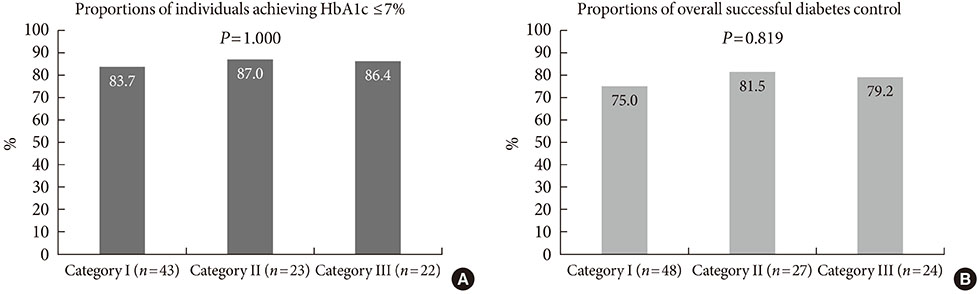
Among 116 subjects, 99 subjects completed the study, with 88 subjects maintaining the initial medication. While each of the metformin-based dual therapies showed a significant decrease in HbA1c (group 1, 8.9% to 6.4%; group 2, 9.0% to 6.6%; group 3, 9.3% to 6.3%; P<0.001 for each), there was no significant difference in the magnitude of HbA1c change among the groups. While the three HbA1c categories showed significantly different baseline HbA1c levels (8.2% vs. 9.9% vs. 11.9%; P<0.001), endpoint HbA1c was not different (6.4% vs. 6.6% vs. 6.0%; P=0.051).
CONCLUSION
The three dual therapies using a combination of metformin and either sulfonylurea, pioglitazone, or sitagliptin showed similar glycemic effectiveness among drug-naive Korean T2D patients. In addition, these regimens were similarly effective across a wide range of baseline HbA1c levels.
MeSH Terms
Figure
Cited by 1 articles
-
Acarbose Add-on Therapy in Patients with Type 2 Diabetes Mellitus with Metformin and Sitagliptin Failure: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study
Hae Kyung Yang, Seung-Hwan Lee, Juyoung Shin, Yoon-Hee Choi, Yu-Bae Ahn, Byung-Wan Lee, Eun Jung Rhee, Kyung Wan Min, Kun-Ho Yoon
Diabetes Metab J. 2019;43(3):287-301. doi: 10.4093/dmj.2018.0054.
Reference
-
1. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998; 352:854–865.2. Holman RR, Paul SK, Bethel MA, Neil HA, Matthews DR. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med. 2008; 359:1565–1576.3. Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995; 28:103–117.4. Rodbard HW, Jellinger PS, Davidson JA, Einhorn D, Garber AJ, Grunberger G, Handelsman Y, Horton ES, Lebovitz H, Levy P, Moghissi ES, Schwartz SS. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009; 15:540–559.5. Ko SH, Kim SR, Kim DJ, Oh SJ, Lee HJ, Shim KH, Woo MH, Kim JY, Kim NH, Kim JT, Kim CH, Kim HJ, Jeong IK, Hong EK, Cho JH, Mok JO, Yoon KH. Committee of Clinical Practice Guidelines, Korean Diabetes Association. 2011 clinical practice guidelines for type 2 diabetes in Korea. Diabetes Metab J. 2011; 35:431–436.6. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. American Diabetes Association (ADA). European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012; 35:1364–1379.7. Yoon KH, Shin JA, Kwon HS, Lee SH, Min KW, Ahn YB, Yoo SJ, Ahn KJ, Park SW, Lee KW, Sung YA, Park TS, Kim MS, Kim YK, Nam MS, Kim HS, Park Ie B, Park JS, Woo JT, Son HY. Comparison of the efficacy of glimepiride, metformin, and rosiglitazone monotherapy in Korean drug-naive type 2 diabetic patients: the practical evidence of antidiabetic monotherapy study. Diabetes Metab J. 2011; 35:26–33.8. Bennett WL, Maruthur NM, Singh S, Segal JB, Wilson LM, Chatterjee R, Marinopoulos SS, Puhan MA, Ranasinghe P, Block L, Nicholson WK, Hutfless S, Bass EB, Bolen S. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011; 154:602–613.9. Bennett WL, Balfe LM, Faysal JM. AHRQ's comparative effectiveness research on oral medications for type 2 diabetes: a summary of the key findings. J Manag Care Pharm. 2012; 18:1 Suppl A. 1–22.10. Garber AJ, Donovan DS Jr, Dandona P, Bruce S, Park JS. Efficacy of glyburide/metformin tablets compared with initial monotherapy in type 2 diabetes. J Clin Endocrinol Metab. 2003; 88:3598–3604.11. Rosenstock J, Rood J, Cobitz A, Biswas N, Chou H, Garber A. Initial treatment with rosiglitazone/metformin fixed-dose combination therapy compared with monotherapy with either rosiglitazone or metformin in patients with uncontrolled type 2 diabetes. Diabetes Obes Metab. 2006; 8:650–660.12. Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE. Sitagliptin 036 Study Group. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007; 30:1979–1987.13. Tosi F, Muggeo M, Brun E, Spiazzi G, Perobelli L, Zanolin E, Gori M, Coppini A, Moghetti P. Combination treatment with metformin and glibenclamide versus single-drug therapies in type 2 diabetes mellitus: a randomized, double-blind, comparative study. Metabolism. 2003; 52:862–867.14. Kaku K. Efficacy and safety of therapy with metformin plus pioglitazone in the treatment of patients with type 2 diabetes: a double-blind, placebo-controlled, clinical trial. Curr Med Res Opin. 2009; 25:1111–1119.15. Rhee EJ, Lee WY, Min KW, Shivane VK, Sosale AR, Jang HC, Chung CH, Nam-Goong IS, Kim JA, Kim SW. Gemigliptin Study 006 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor gemigliptin compared with sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Obes Metab. 2013; 15:523–530.16. Rhee SY, Woo JT. The prediabetic period: review of clinical aspects. Diabetes Metab J. 2011; 35:107–116.17. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004; 27:1487–1495.18. Bloomgarden ZT, Dodis R, Viscoli CM, Holmboe ES, Inzucchi SE. Lower baseline glycemia reduces apparent oral agent glucose-lowering efficacy: a meta-regression analysis. Diabetes Care. 2006; 29:2137–2139.19. Deacon CF. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes Metab. 2011; 13:7–18.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Metformin Based Dual-Combination Therapies in Drug Naive Type 2 Diabetic Patients
- Antihyperglycemic agent combination therapy for patients with type 2 diabetes mellitus
- Two-Year Therapeutic Efficacy and Safety of Initial Triple Combination of Metformin, Sitagliptin, and Empagliflozin in Drug-Naïve Type 2 Diabetes Mellitus Patients
- Correction to: “Cardiovascular Outcomes Comparison of Dipeptidyl Peptidase-4 Inhibitors Versus Sulfonylurea as Add-on Therapy for Type 2 Diabetes Mellitus: A Meta-Analysis”
- Predictors of the Therapeutic Efficacy and Consideration of the Best Combination Therapy of Sodium-Glucose Co-transporter 2 Inhibitors