Ann Dermatol.
2011 Dec;23(Suppl 3):S314-S318. 10.5021/ad.2011.23.S3.S314.
Photosensitivity Reactions to Vandetanib: Redevelopment after Sequential Treatment with Docetaxel
- Affiliations
-
- 1Department of Dermatology, Gachon University of Medicine and Science, Gil Hospital, Incheon, Korea. dmjj1@gilhospital.com
- 2Division of Hematology and Oncology, Department of Internal Medicine, Gachon University of Medicine and Science, Gil Hospital, Incheon, Korea.
- KMID: 2171860
- DOI: http://doi.org/10.5021/ad.2011.23.S3.S314
Abstract
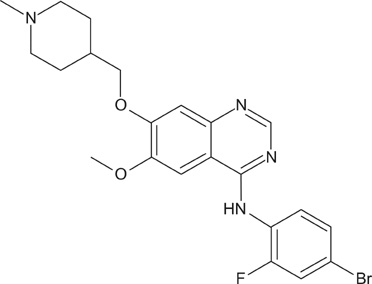
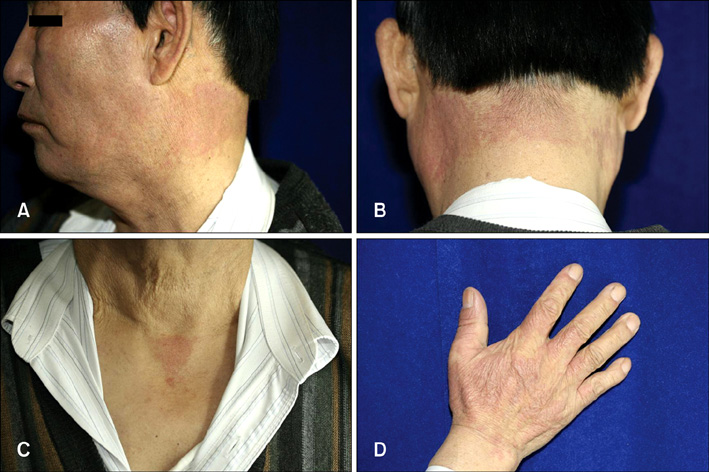
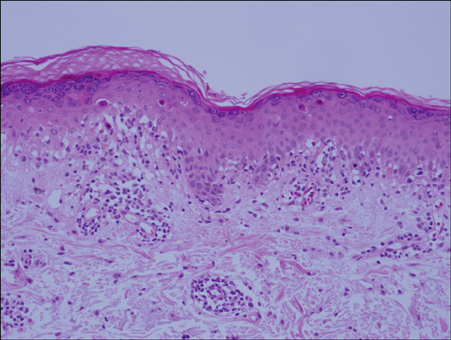
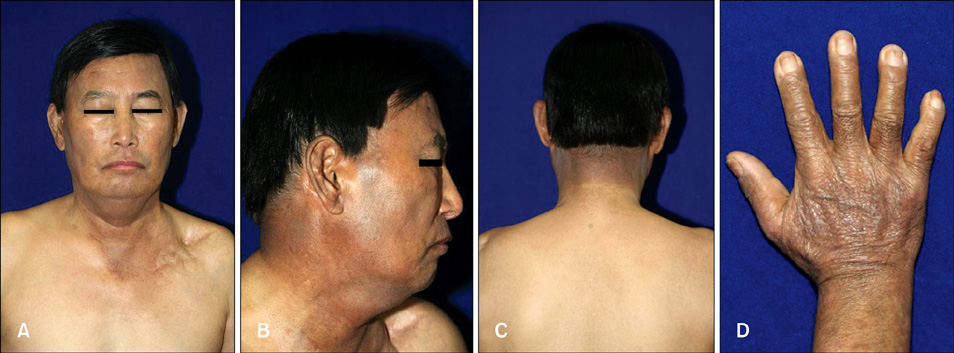
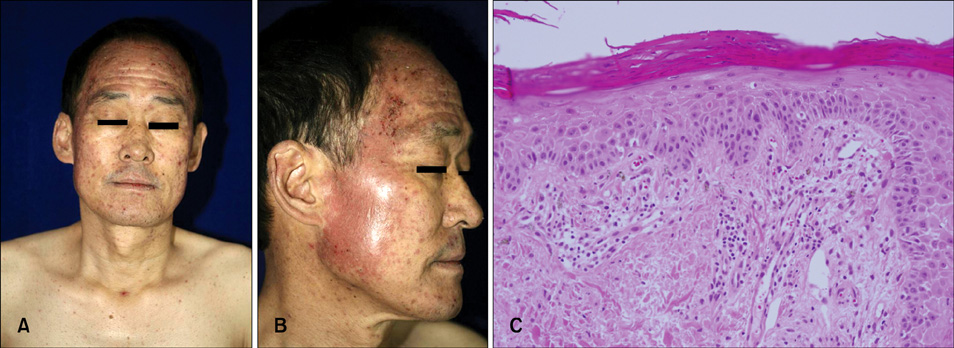
- Vandetanib (ZD6474, Zactima(TM)) is a novel, orally available inhibitor of different intracellular signaling pathways involved in tumor growth, progression, and angiogenesis, including vascular endothelial growth factor receptor-2, epidermal growth factor receptor, and rearranged during transfection tyrosine kinase activity. The most frequently reported adverse events attributed to vandetanib include diarrhea, elevated aminotransferase, asymptomatic corrected QC interval prolongation, and hypertension. In a few randomized, double-blinded studies, cutaneous adverse events including these general symptoms have been reported, but there are only a few reports on the photosensitivity reaction to vandetanib domestically as conducted by dermatologists. In this report, we describe two cases of photosensitivity reactions induced by vandetanib. After improvement with steroid and antihistamine, the photosensitivity reaction was redeveloped by sequential treatment with docetaxel.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Pirfenidone-Induced Photosensitivity in a Patient with Idiopathic Pulmonary Fibrosis
Ho-Jin Kim, Jeong-Wan Seo, Tae-Hoon Kim, Seung-Hwan Choi, Ki-Hoon Song, Ki-Ho Kim
Ann Dermatol. 2018;30(5):614-616. doi: 10.5021/ad.2018.30.5.614.
Reference
-
1. James WD, Berger TG, Elston DM. Andrews' diseases of the skin. 2006. 10th ed. Philadelphia: WB Saunders;32–33.2. Henry WL. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Abnormal responses to ultraviolet radiation: photosensitivity induced by exogenous agents. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;828–834.3. Hölzle E, Lehmann P, Neumann N. Phototoxic and photoallergic reactions. J Dtsch Dermatol Ges. 2009. 7:643–649.
Article4. Stein KR, Scheinfeld NS. Drug-induced photoallergic and phototoxic reactions. Expert Opin Drug Saf. 2007. 6:431–443.
Article5. Racette AJ, Roenigk HH Jr, Hansen R, Mendelson D, Park A. Photoaging and phototoxicity from long-term voriconazole treatment in a 15-year-old girl. J Am Acad Dermatol. 2005. 52:5 Suppl 1. S81–S85.
Article6. Heymach JV. ZD6474--clinical experience to date. Br J Cancer. 2005. 92:Suppl 1. S14–S20.7. Sathornsumetee S, Rich JN. Vandetanib, a novel multitargeted kinase inhibitor, in cancer therapy. Drugs Today (Barc). 2006. 42:657–670.8. Gridelli C, Maione P, Rossi A, Falanga M, Bareschino M, Schettino C, et al. New avenues for second-line treatment of metastatic non-small-cell lung cancer. Expert Rev Anticancer Ther. 2009. 9:115–124.
Article9. Engels FK, Mathot RA, Verweij J. Alternative drug formulations of docetaxel: a review. Anticancer Drugs. 2007. 18:95–103.
Article10. Baker J, Ajani J, Scotté F, Winther D, Martin M, Aapro MS, et al. Docetaxel-related side effects and their management. Eur J Oncol Nurs. 2009. 13:49–59.
Article11. Lankerani L, Baron ED. Photosensitivity to exogenous agents. J Cutan Med Surg. 2004. 8:424–431.
Article12. Moore DE. Drug-induced cutaneous photosensitivity: incidence, mechanism, prevention and management. Drug Saf. 2002. 25:345–372.13. González E, González S. Drug photosensitivity, idiopathic photodermatoses, and sunscreens. J Am Acad Dermatol. 1996. 35:871–885.
Article14. Elder D, Elenitsas R, Johnson B Jr, Murphy G, Xu Xl, Xu X. Lever's histopathology of the skin. 2009. 10th ed. Philadelphia: Lippincott-Raven;318–319.15. Holden SN, Eckhardt SG, Basser R, de Boer R, Rischin D, Green M, et al. Clinical evaluation of ZD6474, an orally active inhibitor of VEGF and EGF receptor signaling, in patients with solid, malignant tumors. Ann Oncol. 2005. 16:1391–1397.
Article16. Tamura T, Minami H, Yamada Y, Yamamoto N, Shimoyama T, Murakami H, et al. A phase I dose-escalation study of ZD6474 in Japanese patients with solid, malignant tumors. J Thorac Oncol. 2006. 1:1002–1009.
Article17. Kiura K, Nakagawa K, Shinkai T, Eguchi K, Ohe Y, Yamamoto N, et al. A randomized, double-blind, phase IIa dose-finding study of Vandetanib (ZD6474) in Japanese patients with non-small cell lung cancer. J Thorac Oncol. 2008. 3:386–393.
Article18. Miller KD, Trigo JM, Wheeler C, Barge A, Rowbottom J, Sledge G, et al. A multicenter phase II trial of ZD6474, a vascular endothelial growth factor receptor-2 and epidermal growth factor receptor tyrosine kinase inhibitor, in patients with previously treated metastatic breast cancer. Clin Cancer Res. 2005. 11:3369–3376.
Article19. Chew L, Chuen VS. Cutaneous reaction associated with weekly docetaxel administration. J Oncol Pharm Pract. 2009. 15:29–34.
Article20. Kong HH, Fine HA, Stern JB, Turner ML. Cutaneous pigmentation after photosensitivity induced by vandetanib therapy. Arch Dermatol. 2009. 145:923–925.
Article