Ann Dermatol.
2011 Dec;23(Suppl 3):S343-S345. 10.5021/ad.2011.23.S3.S343.
Stevens-Johnson Syndrome Induced by Vandetanib
- Affiliations
-
- 1Department of Dermatology, Institute of Health Science, School of Medicine, Gyeongsang National University, Jinju, Korea. dr_chiny@hotmail.com
- 2Department of Dermatology, School of Medicine, Kangwon National University, Chuncheon, Korea.
- KMID: 2171868
- DOI: http://doi.org/10.5021/ad.2011.23.S3.S343
Abstract
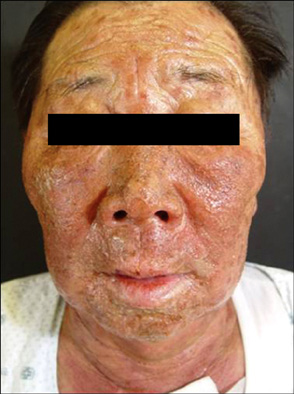
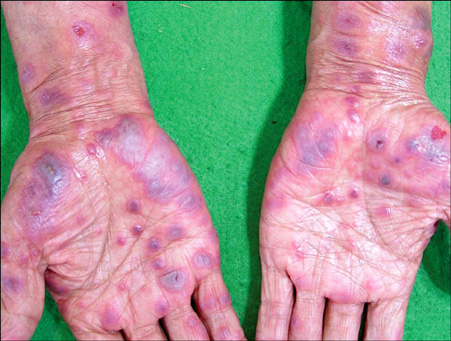
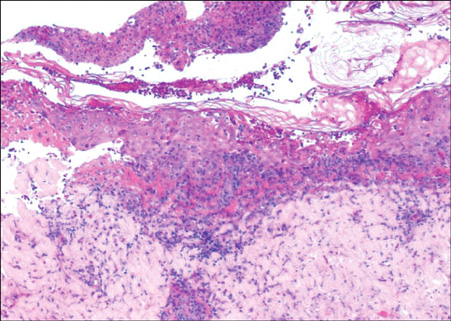
- Vandetanib is a once-daily oral anticancer drug that selectively inhibits key signaling pathways in cancer by targeting vascular endothelial growth factor receptors, epidermal growth factor receptors tyrosine kinase, and rearranged during transfection-dependent tumor cell proliferation and survival. The most frequently reported adverse events attributed to vandetanib include diarrhea, elevated aminotransferase, asymptomatic corrected QT interval prolongation, and hypertension. Though a number of randomized, doubleblind studies, including cutaneous adverse events attributed to vandetanib, have been reported along with these general symptoms, no case of Stevens-Johnson syndrome (SJS) has been reported. This paper demonstrates a case of SJS induced by vandetanib.
MeSH Terms
-
Carcinoma, Non-Small-Cell Lung
Cell Proliferation
Diarrhea
Hypertension
Piperidines
Protein-Tyrosine Kinases
Quinazolines
Receptor, Epidermal Growth Factor
Receptors, Vascular Endothelial Growth Factor
Stevens-Johnson Syndrome
Piperidines
Protein-Tyrosine Kinases
Quinazolines
Receptor, Epidermal Growth Factor
Receptors, Vascular Endothelial Growth Factor
Figure
Cited by 1 articles
-
A Case of Stevens-Johnson Syndrome Probably Induced by Herbal Medicine
Ji Hong Lim, Sang Hyun Cho, Jeong Deuk Lee, Hei Sung Kim
Ann Dermatol. 2018;30(4):481-483. doi: 10.5021/ad.2018.30.4.481.
Reference
-
1. Heymach JV. ZD6474--clinical experience to date. Br J Cancer. 2005. 92:Suppl 1. S14–S20.2. Horti J, Widmark A, Stenzl A, Federico MH, Abratt RP, Sanders N, et al. A randomized, double-blind, placebocontrolled phase II study of vandetanib plus docetaxel/prednisolone in patients with hormone-refractory prostate cancer. Cancer Biother Radiopharm. 2009. 24:175–180.
Article3. Wells SA Jr, Gosnell JE, Gagel RF, Moley J, Pfister D, Sosa JA, et al. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol. 2010. 28:767–772.
Article4. Auquier-Dunant A, Mockenhaupt M, Naldi L, Correia O, Schröder W, Roujeau JC. SCAR Study Group. Severe Cutaneous Adverse Reactions. Correlations between clinical patterns and causes of erythema multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis: results of an international prospective study. Arch Dermatol. 2002. 138:1019–1024.5. Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993. 129:92–96.
Article6. Annunziata CM, Walker AJ, Minasian L, Yu M, Kotz H, Wood BJ, et al. Vandetanib, designed to inhibit VEGFR2 and EGFR signaling, had no clinical activity as monotherapy for recurrent ovarian cancer and no detectable modulation of VEGFR2. Clin Cancer Res. 2010. 16:664–672.
Article7. AstraZeneca core presentation: Vandetanib for unresectable locally advanced or metastatic MTC, Antoine Yver. accessed January 2011. www.fda.gov.8. FDA Briefing Document Oncologic Drugs Advisory Committee Meeting. 2010. 12. 02. accessed December 2010. NDA 22405/S000 Vandetanib www.fda.gov.9. Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCARstudy. J Invest Dermatol. 2008. 128:35–44.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Five Cases of Stevens-Johnson Syndrome May Be Associated with Methazolamide Treatment
- Clinical Study of Stevens-Johnson Syndrome
- A Case of Stevens-Johnson Syndrome in a Hypoxic Brain Injury Patient Treated with Carbamazepine
- A Case of Secondary Milia Associated with Stevens-Johnson Syndrome
- A Case of High Dose Cytosine Arabinoside Induced Stevens-Johnson Syndrome in a Patient with Malignant Lymphoma