Allergy Asthma Respir Dis.
2017 Nov;5(6):358-360. 10.4168/aard.2017.5.6.358.
Photosensitivity caused by dronedarone: A case report
- Affiliations
-
- 1Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, Korea. drsys93@naver.com
- 2Department of Dermatology, Ajou University School of Medicine, Suwon, Korea.
- KMID: 2396678
- DOI: http://doi.org/10.4168/aard.2017.5.6.358
Abstract
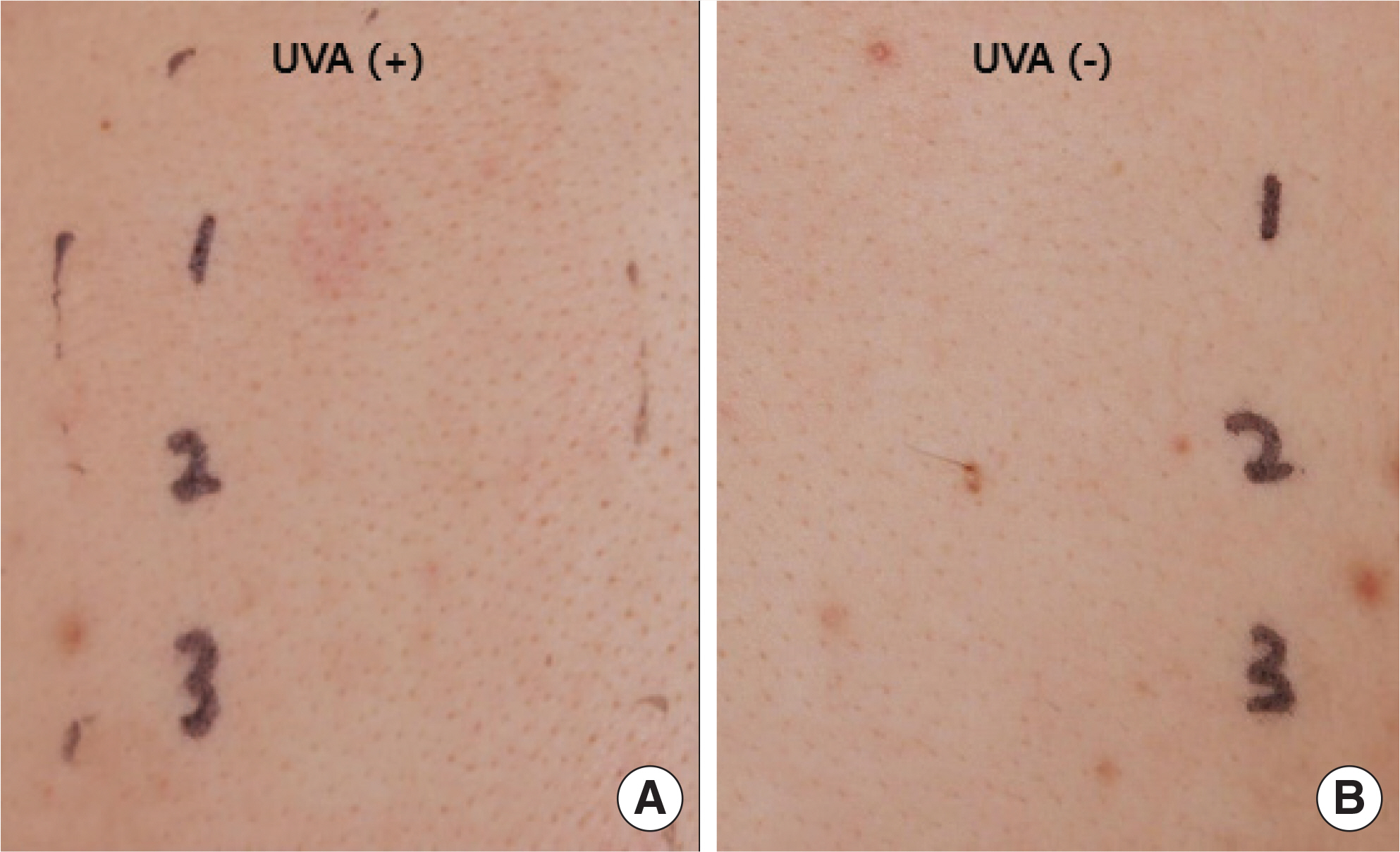
- Dronedarone is a new antiarrhythmic drug for the treatment of nonpermanent atrial fibrillation. Compared with amiodarone, it is regarded as a safe medication due to its structural differences. In this report, we describe a 56-year-old man who developed photosensitivity due to dronedarone. He presented with itchy skin rashes for 1 week. Maculopapular exanthema was localized on the neck, both arms, and both hands, with sparing of the other parts of the body. Dronedarone was prescribed 4 weeks ago when atrial fibrillation occurred. After development of skin rashes, dronedarone was discontinued, and systemic steroid, antihistamine, and topical corticosteroid were administered for 1 week, with improvement in skin rashes. The photopatch test was performed with antiarrhythmic drugs, including dronedarone, amiodarone, and flecainide, 4 weeks after withdrawal of dronedarone. Positive reactions were recorded only to dronedarone at the site exposed to ultraviolet A. He was diagnosed with dronedarone-induced photosensitivity and advised to change the antiarrhythmic medication to others. There have been a few case reports on photosensitivity reactions due to dronedarone, which were diagnosed only by clinical suspicion. However, we suspected photosensitivity and proved it by the photopatch test. Photosensitivity should be considered in patients having skin rashes on the exposed area and taking antiarrhythmic medication, including dronedarone.
Keyword
MeSH Terms
Figure
Reference
-
1. Selvaag E. Clinical drug photosensitivity. A retrospective analysis of reports to the Norwegian Adverse Drug Reactions Committee from the years 1970-1994. Photodermatol Photoimmunol Photomed. 1997; 13:21–3.
Article2. Glatz M, Hofbauer GF. Phototoxic and photoallergic cutaneous drug reactions. Chem Immunol Allergy. 2012; 97:167–79.
Article3. Monteiro AF, Rato M, Martins C. Drug-induced photosensitivity: photo-allergic and phototoxic reactions. Clin Dermatol. 2016; 34:571–81.
Article4. Onoue S, Seto Y, Sato H, Nishida H, Hirota M, Ashikaga T, et al. Chemical photoallergy: photobiochemical mechanisms, classification, and risk assessments. J Dermatol Sci. 2017; 85:4–11.
Article5. Vamos M, Hohnloser SH. Amiodarone and dronedarone: an update. Trends Cardiovasc Med. 2016; 26:597–602.
Article6. De Ferrari GM, Dusi V. Drug safety evaluation of dronedarone in atrial fibrillation. Expert Opin Drug Saf. 2012; 11:1023–45.
Article7. Gecks T, Prochnau D, Franz M, Jung C, Kühnert H, Schliemann S, et al. Toxic epidermal necrolysis during dronedarone treatment: first report of a severe serious adverse event of a new antiarrhythmic drug. Cardiovasc Toxicol. 2015; 15:399–401.
Article8. Kuo S, Menon K, Kundu RV. Photosensitivity reaction from dronedarone for atrial fibrillation. Cutis. 2014; 94:E10–1.9. Ladizinski B, Elpern DJ. Dronaderone-induced phototoxicity. J Drugs Dermatol. 2013; 12:946–7.10. Kutlubay Z, Sevim A, Engin B, Tüzün Y. Photodermatoses, including phototoxic and photoallergic reactions (internal and external). Clin Dermatol. 2014; 32:73–9.
Article11. Wilm A, Berneburg M. Photoallergy. J Dtsch Dermatol Ges. 2015; 13:7–12.
Article