Comparison of Antiretroviral Regimens: Adverse Effects and Tolerability Failure that Cause Regimen Switching
- Affiliations
-
- 1Division of Allergy and Infectious Diseases, Department of Internal Medicine, Kyungpook National University Hospital, Daegu, Korea. ksw2kms@knu.ac.kr
- KMID: 2170500
- DOI: http://doi.org/10.3947/ic.2015.47.4.231
Abstract
- BACKGROUND
The efficacy of antiretroviral therapy (ART) has improved, and the adverse effects of antiretroviral drugs have been reduced. However, these adverse effects still significantly influence patient compliance, increasing the risk of tolerability failure. Therefore, we investigated the adverse effects and tolerability failure causing changes in the first ART regimen, and identified the regimens that were most vulnerable to switching.
MATERIALS AND METHODS
We enrolled patients with human immunodeficiency virus (HIV) who commenced their first ART between January 1, 2011 and July 30, 2014. Patients who started their first ART regimen at the Kyungpook National University Hospital were included in the study if they were aged > or =18 years and were followed-up for > or =12 weeks. The primary dependent variable was the duration of treatment on the same ART regimen. We analyzed the maintenance rate of the first ART regimen based on the treatment duration between these groups using survival analysis and log rank test. The frequency of the adverse effects of ART regimens was analyzed by multiple response data analysis.
RESULTS
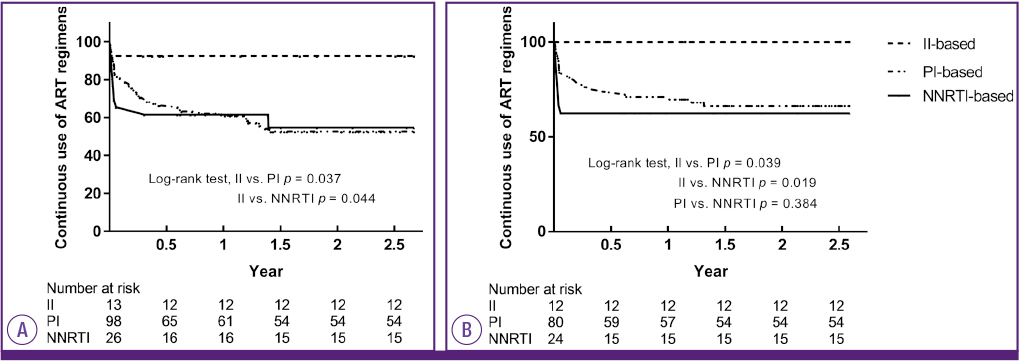
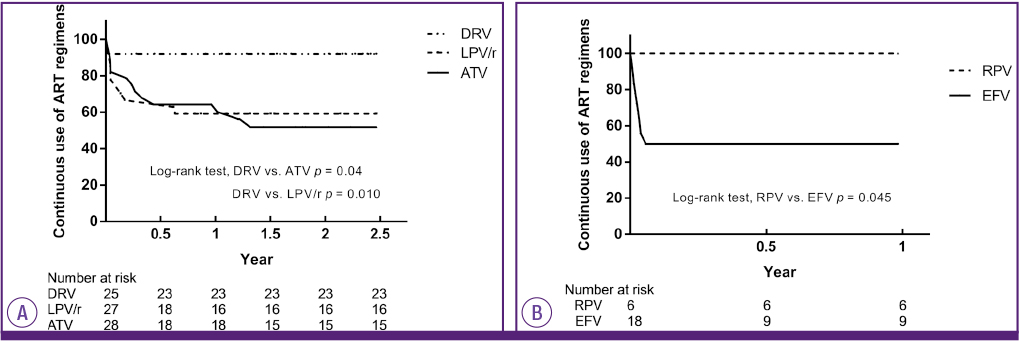
During the investigation period, 137 patients were enrolled. Eighty-one patients were maintained on the initial treatment regimen (59.1%). In protease inhibitor (PI)-based regimen group, 54 patients were maintained on the initial treatment regimen (54/98, 55.1%). In non-nucleoside reverse transcriptase inhibitor (NNRTI)-and integrase inhibitor (II)-based regimen group, 15 (15/26, 57.7%) and 12 (12/13, 92.3%) patients were maintained on the initial treatment regimen, respectively. Adverse effects that induced ART switching included rash (16/35, 45.7%), gastrointestinal discomfort or pain (7/35, 20%), diarrhea (7/35, 20%), hyperbilirubinemia (6/35, 17.1%), headache or dizziness (3/35, 8.5%). Among the treatment regimens, the group receiving an II-based regimen showed the least switching. The group receiving PI-and NRTI-based regimens were most likely to switch due to adverse effects during the early treatment period. However, after about 18 months, switching was rarely observed in these groups. Among the PI drugs, darunavir/ritonavir showed fewer drug changes than atazanavir/ritonavir (P = 0.004, log rank test) and lopinavir/ritonavir (P = 0.010). Among the NNRTI drugs, rilpivirne produced less switching than efavirenz (P = 0.045).
CONCLUSION
Adverse effects to ART resulted in about a quarter of patients switching drugs during the early treatment period. II-based regimens were advantageous because they were less likely to induce switching within 18 months of treatment commencement. These findings indicated the importance of considering and monitoring the adverse effects of ART in order to improve adherence.
MeSH Terms
Figure
Cited by 4 articles
-
Causes of HIV Drug Non-Adherence in Korea: Korea HIV/AIDS Cohort Study, 2006-2015
Min Jung Kim, Sang Ah Lee, Hyun-Ha Chang, Min Ja Kim, Jun Hee Woo, Sang Il Kim, Chun Kang, Mee-Kyung Kee, Ju-yeon Choi, Yunsu Choi, Bo Youl Choi, June Myung Kim, Jun Yong Choi, Hyo Youl Kim, Joon-Young Song, Shin-Woo Kim,
Infect Chemother. 2017;49(3):213-218. doi: 10.3947/ic.2017.49.3.213.Safety and Efficacy of Ziagen (Abacavir Sulfate) in HIV-Infected Korean Patients
Heawon Ann, Ki-Hyon Kim, Hyun-Young Choi, Hyun-Ha Chang, Sang Hoon Han, Kye-Hyung Kim, Jin-Soo Lee, Yeon-Sook Kim, Kyung-Hwa Park, Young Keun Kim, Jang Wook Sohn, Na-Ra Yun, Chang-Seop Lee, Young Wha Choi, Yil-Seob Lee, Shin-Woo Kim
Infect Chemother. 2017;49(3):205-212. doi: 10.3947/ic.2017.49.3.205.Safety and Effectiveness Analysis of Kivexa® (lamivudine/abacavir sulfate) in Human Immunodeficiency Virus Infected Korean Patients
Heawon Ann, Yil-Seob Lee, Yeon-Sook Kim, Sook-In Jung, Sun-Hee Lee, Chang-Seop Lee, Jin-Soo Lee, Won Suk Choi, Young Hwa Choi, Shin-Woo Kim
Infect Chemother. 2019;51(2):150-160. doi: 10.3947/ic.2019.51.2.150.Effectiveness, Safety, and Tolerability of a Switch to Dual Therapy with Dolutegravir Plus Cobicistat-Boosted Darunavir in Treatment-Experienced Patients with Human Immunodeficiency Virus
Sang-Ah Lee, Shin-Woo Kim, Hyun-Ha Chang, Hyejin Jung, Yoonjung Kim, Soyoon Hwang, Sujeong Kim, Han-Ki Park, Jong-Myung Lee
Infect Chemother. 2018;50(3):252-262. doi: 10.3947/ic.2018.50.3.252.
Reference
-
1. Mocroft A, Ledergerber B, Katlama C, Kirk O, Reiss P, d'Arminio Monforte A, Knysz B, Dietrich M, Phillips AN, Lundgren JD. EuroSIDA study group. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003; 362:22–29.
Article2. Brady MT, Oleske JM, Williams PL, Elgie C, Mofenson LM, Dankner WM, Van Dyke RB. Pediatric AIDS Clinical Trials Group219/219C Team. Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. J Acquir Immune Defic Syndr. 2010; 53:86–94.
Article3. Max B, Sherer R. Management of the adverse effects of antiretroviral therapy and medication adherence. Clin Infect Dis. 2000; 30:Suppl 2. S96–S116.
Article4. Bangsberg DR, Perry S, Charlebois ED, Clark RA, Roberston M, Zolopa AR, Moss A. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001; 15:1181–1183.
Article5. Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med. 2007; 146:564–573.
Article6. The Korean Society for AIDS. Clinical guidelines for the diagnosis and treatment of HIV/AIDS in HIV-infected Koreans. Infect Chemother. 2011; 43:89–128.7. Chesney MA. Factors affecting adherence to antiretroviral therapy. Clin Infect Dis. 2000; 30:Suppl 2. S171–S176.
Article8. Lennox JL, Landovitz RJ, Ribaudo HJ, Ofotokun I, Na LH, Godfrey C, Kuritzkes DR, Sagar M, Brown TT, Cohn SE, McComsey GA, Aweeka F, Fichtenbaum CJ, Presti RM, Koletar SL, Haas DW, Patterson KB, Benson CA, Baugh BP, Leavitt RY, Rooney JF, Seekins D, Currier JS. ACTG A5257 Team. Efficacy and tolerability of 3 nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naive volunteers infected with HIV-1: a randomized, controlled equivalence trial. Ann Intern Med. 2014; 161:461–471.
Article9. Dennis L, Kasper Anthony S, Fauci Stephen L, Hauser Dan L, Longo J, Larry Jameson Jeseph Loscalzo. Harrison's Principles of Internal Medicine. 19 ed. McGraw-Hill Education;2015.10. Thurairajah PH, Thorburn D, Hubscher S, White A, Lai WK, O'Donnell K, Mutimer D. Incidence and characterization of serum transaminases elevations in pegylated interferon and ribavirin treated patients with chronic hepatitis C. Aliment Pharmacol Ther. 2007; 25:1293–1300.
Article11. National Institute of Allergy and Infectious Diseases. Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events. Rockville, MD: National Institute of Allergy and Infectious Diseases;2004.12. Group AIDSCT. Table of Grading Severity of Adult Adverse Experiences. Rockville, MD: US Division of AIDS, National Institute of Allergy and Infectious Disease;1996.13. American Diabetes Association. Standards of medical care in diabetes-2015 abridged for primary care providers. Clin Diabetes. 2015; 33:97–111.14. Hawkins T. Understanding and managing the adverse effects of antiretroviral therapy. Antiviral Res. 2010; 85:201–209.
Article15. Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998; 338:853–860.
Article16. Mocroft A, Youle M, Moore A, Sabin CA, Madge S, Lepri AC, Tyrer M, Chaloner C, Wilson D, Loveday C, Johnson MA, Phillips AN. Reasons for modification and discontinuation of antiretrovirals: results from a single treatment centre. AIDS. 2001; 15:185–194.
Article17. Mocroft A, Phillips AN, Soriano V, Rockstroh J, Blaxhult A, Katlama C, Boron-Kaczmarska A, Viksna L, Kirk O, Lundgren JD. EuroSIDA Study Group. Reasons for stopping antiretrovirals used in an initial highly active antiretroviral regimen: increased incidence of stopping due to toxicity or patient/physician choice in patients with hepatitis C coinfection. AIDS Res Hum Retroviruses. 2005; 21:743–752.
Article18. Cicconi P, Cozzi-Lepri A, Castagna A, Trecarichi EM, Antinori A, Gatti F, Cassola G, Sighinolfi L, Castelli P, d'Arminio Monforte A. ICoNA Foundation Study Group. Insights into reasons for discontinuation according to year of starting first regimen of highly active antiretroviral therapy in a cohort of antiretroviral-naïve patients. HIV Med. 2010; 11:104–113.
Article19. d'Arminio Monforte A, Lepri AC, Rezza G, Pezzotti P, Antinori A, Phillips AN, Angarano G, Colangeli V, De Luca A, Ippolito G, Caggese L, Soscia F, Filice G, Gritti F, Narciso P, Tirelli U, Moroni M. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naïve patients. I.CO.N.A. Study Group. Italian Cohort of Antiretroviral-Naïve Patients. AIDS. 2000; 14:499–507.20. Al-Dakkak I, Patel S, McCann E, Gadkari A, Prajapati G, Maiese EM. The impact of specific HIV treatment-related adverse events on adherence to antiretroviral therapy: a systematic review and meta-analysis. AIDS Care. 2013; 25:400–414.
Article21. O'Brien ME, Clark RA, Besch CL, Myers L, Kissinger P. Patterns and correlates of discontinuation of the initial HAART regimen in an urban outpatient cohort. J Acquir Immune Defic Syndr. 2003; 34:407–414.22. Orkin C, DeJesus E, Khanlou H, Stoehr A, Supparatpinyo K, Lathouwers E, Lefebvre E, Opsomer M, Van de Casteele T, Tomaka F. Final 192-week efficacy and safety of once-daily darunavir/ritonavir compared with lopinavir/ritonavir in HIV-1-infected treatment-naïve patients in the ARTEMIS trial. HIV Med. 2013; 14:49–59.
Article23. Molina JM, Andrade-Villanueva J, Echevarria J, Chetchotisakd P, Corral J, David N, Moyle G, Mancini M, Percival L, Yang R, Wirtz V, Lataillade M, Absalon J, McGrath D. CASTLE Study Team. Once-daily atazanavir/ritonavir compared with twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 96-week efficacy and safety results of the CASTLE study. J Acquir Immune Defic Syndr. 2010; 53:323–332.
Article24. Molina JM, Cahn P, Grinsztejn B, Lazzarin A, Mills A, Saag M, Supparatpinyo K, Walmsley S, Crauwels H, Rimsky LT, Vanveggel S, Boven K. ECHO study group. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Lancet. 2011; 378:238–246.
Article25. Cohen CJ, Andrade-Villanueva J, Clotet B, Fourie J, Johnson MA, Ruxrungtham K, Wu H, Zorrilla C, Crauwels H, Rimsky LT, Vanveggel S, Boven K. THRIVE study group. Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): a phase 3, randomised, non-inferiority trial. Lancet. 2011; 378:229–237.
Article26. Lennox JL, DeJesus E, Lazzarin A, Pollard RB, Madruga JV, Berger DS, Zhao J, Xu X, Williams-Diaz A, Rodgers AJ, Barnard RJ, Miller MD, DiNubile MJ, Nguyen BY, Leavitt R, Sklar P. STARTMRK investigators. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009; 374:796–806.
Article27. Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet. 2000; 356:1423–1430.
Article28. Rockstroh JK, DeJesus E, Lennox JL, Yazdanpanah Y, Saag MS, Wan H, Rodgers AJ, Walker ML, Miller M, DiNubile MJ, Nguyen BY, Teppler H, Leavitt R, Sklar P. STARTMRK Investigators. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naive HIV-1-infected patients: final 5-year results from STARTMRK. J Acquir Immune Defic Syndr. 2013; 63:77–85.
Article29. Nishijima T, Komatsu H, Teruya K, Tanuma J, Tsukada K, Gatanaga H, Kikuchi Y, Oka S. Once-daily darunavir/ritonavir and abacavir/lamivudine versus tenofovir/emtricitabine for treatment-naïve patients with a baseline viral load of more than 100 000 copies/ml. AIDS. 2013; 27:839–842.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effectiveness and Tolerability of Dual Therapy with Dolutegravir Plus Darunavir/ cobicistat in Treatment-Experienced Patients with HIV: A 144-Week Follow-Up
- Clinical Implications of New Drugs and Regimens for the Treatment of Drug-resistant Tuberculosis
- Adverse Effects of Antiretroviral Drugs on HIV-infected Koreans
- Effect of Cyclophosphamide and Prednisone as a First-line Treatment for Non-transplant Candidates with Multiple Myeloma
- Comparison of the Efficacy and Tolerability between Same-day Picosulfate and Split-dose Polyethylene Glycol Bowel Preparation for Afternoon Colonoscopy: A Prospective, Randomized, Investigator-blinded Trial