Infect Chemother.
2009 Apr;41(2):95-98. 10.3947/ic.2009.41.2.95.
The First Case of Psoas Muscle Abscess and Sepsis Caused by Actinobacillus ureae in a Chronic Hepatitis B Patient in Korea
- Affiliations
-
- 1Department of Internal Medicine, Eulji University School of Medicine, Daejeon, Korea. yhj822@medimail.co.kr
- KMID: 2170257
- DOI: http://doi.org/10.3947/ic.2009.41.2.95
Abstract
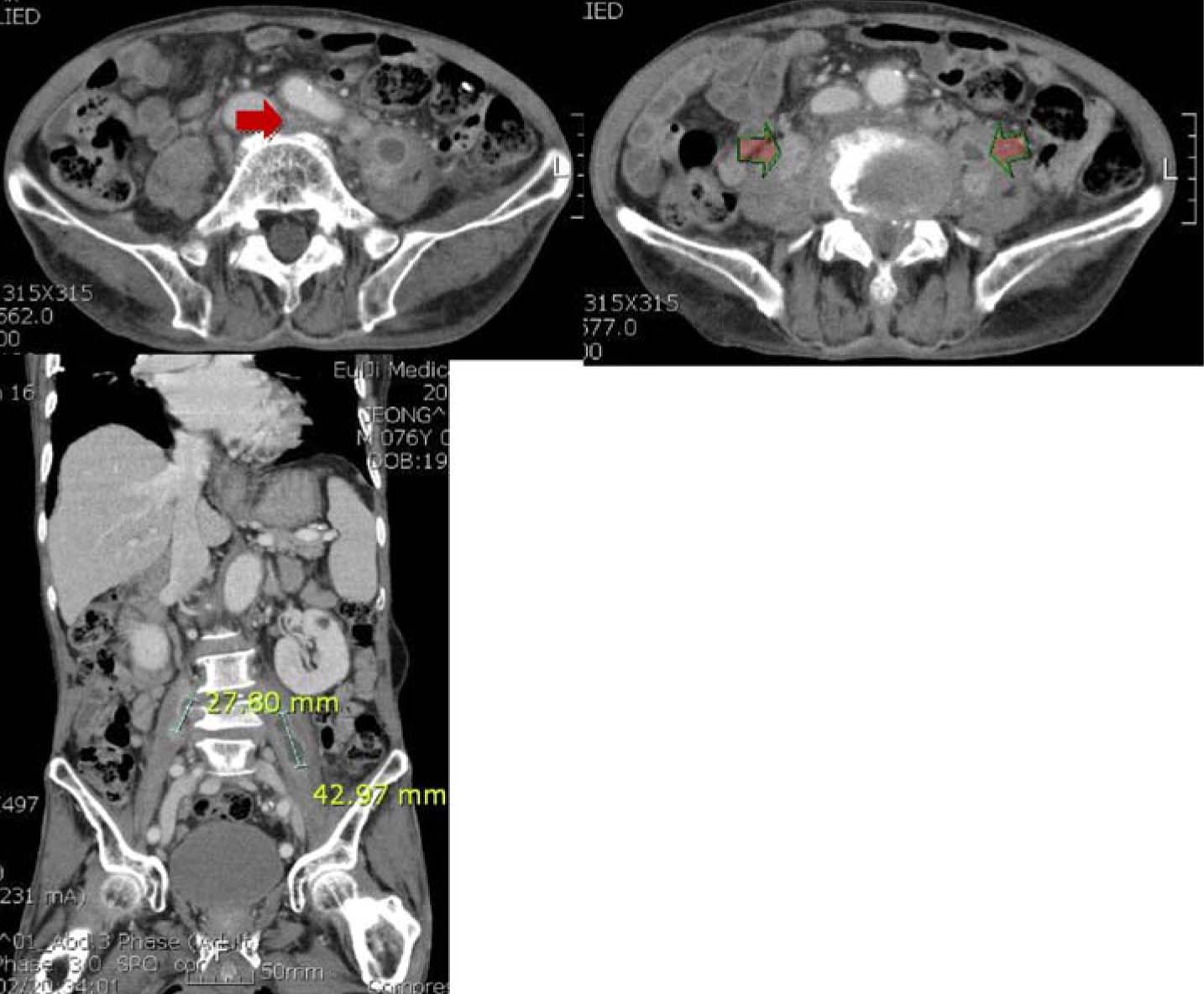
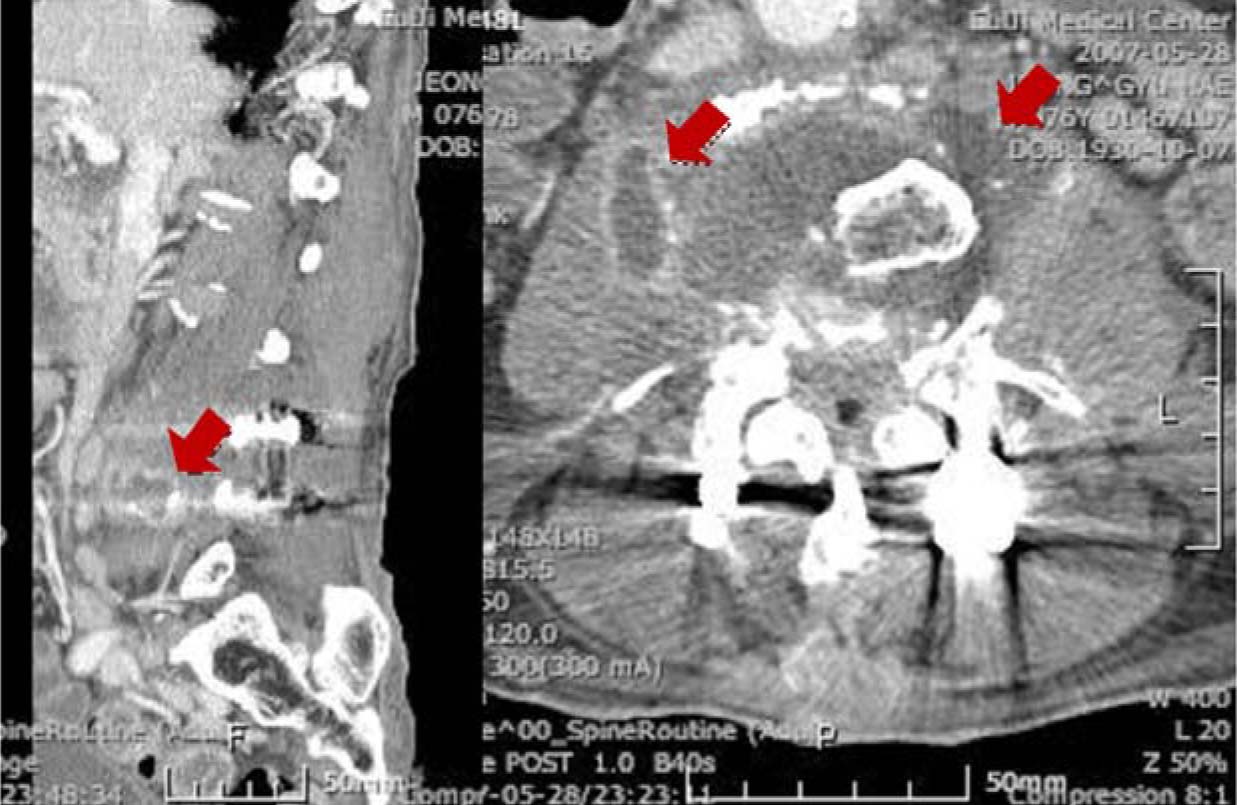
- Actinobacillus ureae, formerly known as Pasteurella ureae, is a rare human pathogen. Twenty-eight cases of A. ureae infections in humans have been reported since its first description in 1960. Various predisposing conditions such as skull fracture, alcohol abuse, neurosurgery, schizophrenia, odontal infection, diabetes, HIV infection/AIDS, Waldenstrom macroglobulinemia, COPD, malnutrition, rheumatoid arthritis, HCV hepatitis, etanercept, or methotrexate have been associated with infections caused by A. ureae. We report the first case, in the medline-based literature, of A. ureae psoas muscle abscess and sepsis in a HBV carrier patient.
Keyword
MeSH Terms
-
Abscess
Actinobacillus
Alcoholism
Arthritis, Rheumatoid
Etanercept
Hepatitis
Hepatitis B, Chronic
Hepatitis, Chronic
HIV
Humans
Immunoglobulin G
Korea
Malnutrition
Methotrexate
Neurosurgery
Pasteurella
Psoas Abscess
Psoas Muscles
Pulmonary Disease, Chronic Obstructive
Receptors, Tumor Necrosis Factor
Schizophrenia
Sepsis
Skull Fractures
Urea
Waldenstrom Macroglobulinemia
Immunoglobulin G
Methotrexate
Receptors, Tumor Necrosis Factor
Urea
Figure
Reference
-
1. Kaka S, Lunz R, Klugman KP. Actinobacillus (Pasteurella) ureae meningitis in a HIV-positive patient. Diagn Microbiol Infect Dis. 1994. 20:105–107.
Article2. de Castro N, Pavie J, Lagrange-Xélot M, Bouvry D, Delisle F, Parrot A, Molina JM. Severe Actinobacillus ureae meningitis in an immunocompromsed patient: Report of one case and review of the literature. Scand J Infect Dis. 2007. 39:1076–1079.
Article3. Starkebaum GA, Plorde JJ. Pasteurella pneumonia: report of a case and review of the literature. J Clin Microbiol. 1977. 5:332–335.
Article4. Maritz FJ, Franco MM, Swart WH. Pasteurella ureae septicaemia. A case report. S Afr Med. J. 1981. 59:53–54.5. Yoshizaki E, Kamiki T, Sakazaki R, Tamura K. A case of bronchopneumonia possibly caused by Pasteurella ureae (author's transl). Kansenshogaku Zasshi. 1981. 55:534–536.6. Pérez JA, de la Iglesia A, de la Iglesia M, Menchero A. Pneumonia caused by Actinobacillus ureae. Enferm Infecc Microbiol Clin. 2000. 18:296–297.7. Gatti F, Seynhaeve V, Weaver R. 1st description of a case of human septicemia due to Pasteurella ureae. Ann Soc Belges Med Trop Parasitol Mycol. 1968. 48:463–468.8. Barardi L, Bourdain J, Chatelain R, Riou J. Diagnostic bacterioligque de Pasteurella ureae: a propos d'un cas de septicemia humaine. Med et Maladies Infect. 1984. 14:36–40.9. Bogaerts J, Lepage P, Kestelyn P, Vandepitte J. Neonatal conjunctivitis caused by Pasteurella ureae. Eur J Clin Microbiol. 1985. 4:427–428.10. Bigel ML, Berardi-Grassias LD, Furioli J. Isolation of Actinobacillus urea (Pasteurella ureae) from a patient with otitis media. Eur J Clin Microbiol Infect Dis. 1988. 7:206–207.
Article11. Noble RC, Marek BJ, Overman SB. Spontaneous bacterial peritonitis caused by Pasteurella ureae. J Clin Microbiol. 1989. 27:375.12. Yamamoto K, Ikeda U, Ogawa C, Fukazawa H, Eto M, Shimada K. Pasteurella ureae endocarditis. Intern Med. 1993. 32:872–874.
Article13. Vay C, Rodríguez C, Sadorin R, Vujacich P, Famiglietti A. Actinobacillus ureae isolated from a patient with chronic bronchitis. Enferm Infecc Microbiol Clin. 1995. 13:569–570.14. Avlami A, Papalambrou C, Tzivra M, Dounis E, Kordossis T. Bone marrow infection caused by Actinobacillus ureae in a rheumatoid arthritis patient. J Infect. 1997. 35:298–299.
Article15. Kaur PP, Derk CT, Chatterji M, Dehoratius RJ. Septic arthritis caused by Actinobacillus ureae in a patient with rheumatoid arthritis receiving anti-tumor necrosis factor-alpha therapy. J Rheumatol. 2004. 31:1663–1665.16. Whitelaw AC, Shankland IM, Elisha BG. Use of 16S rRNA sequencing for identification of Actinobacillus ureae isolated from a cerebrospinal fluid sample. J Clin Microbiol. 2002. 40:666–668.
Article17. Mynter H. Acute pyositis. Buffalo Med Surg J. 1881. 21:202–210.18. Taiwo B. Psoas abscess: a primer for the internist. South Med J. 2001. 94:2–5.19. Ricci MA, Rose FB, Meyer KK. Pyogenic psoas abscess: worldwide variations in etiology. World J Surg. 1986. 10:834–843.
Article20. Drancourt M, Bollet C, Carlioz A, Martelin R, Gayral JP, Raoult D. 16S ribosomal DNA sequence analysis of a large collection of environmental and clinical unidentifiable bacterial isolates. J Clin Microbiol. 2000. 38:3623–3630.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Non-Hodgkin Lymphoma Occurred in Psoas Muscle
- Psoas and Thigh Abscess Caused by Perforated Retrocecal Appendicitis: A Case Report
- The Recurrent Psoas Abscess Caused by Two Different Pathogens: A Case Report
- Ultrasonographic Findings of Psoas abscess and Hematoma
- Psoas Abscess Secondary to Renal Tuberculosis in a Middle-aged Woman