Endocrinol Metab.
2015 Dec;30(4):531-542. 10.3803/EnM.2015.30.4.531.
Economic Evaluation of Recombinant Human Thyroid Stimulating Hormone Stimulation vs. Thyroid Hormone Withdrawal Prior to Radioiodine Ablation for Thyroid Cancer: The Korean Perspective
- Affiliations
-
- 1Division of Endocrinology, Department of Medicine, Myongji Hospital, Seonam University College of Medicine, Goyang, Korea.
- 2Department of Social and Preventive Medicine, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Division of Endocrinology and Metabolism, Department of Medicine, Thyroid Center, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. thyroid@skku.edu
- KMID: 2169663
- DOI: http://doi.org/10.3803/EnM.2015.30.4.531
Abstract
- BACKGROUND
Previous studies have suggested that recombinant human thyroid stimulating hormone (rhTSH) stimulation is an acceptable alternative to thyroid hormone withdrawal (THW) when radioiodine remnant ablation is planned for thyroid cancer treatment, based on superior short-term quality of life with non-inferior remnant ablation efficacy. This study evaluated the cost-effectiveness of radioiodine remnant ablation using rhTSH, compared with the traditional preparation method which renders patients hypothyroid by THW, in Korean perspective.
METHODS
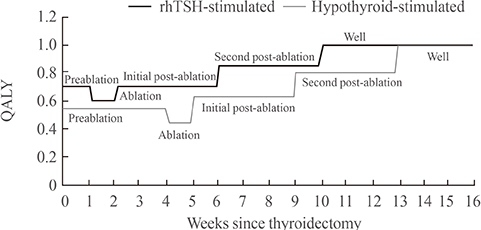
This economic evaluation considered the costs and benefits to the Korean public healthcare system. Clinical experts were surveyed regarding the current practice of radioiodine ablation in Korea and their responses helped inform assumptions used in a cost effectiveness model. Markov modelling with 17 weekly cycles was used to assess the incremental costs per quality-adjusted life year (QALY) associated with rhTSH. Clinical inputs were based on a multi-center, randomized controlled trial comparing remnant ablation success after rhTSH preparation with THW. The additional costs associated with rhTSH were considered relative to the clinical benefits and cost offsets.
RESULTS
The additional benefits of rhTSH (0.036 QALY) are achieved with an additional cost of Korean won 961,105, equating to cost per QALY of 26,697,361. Sensitivity analyses had only a modest impact upon cost-effectiveness, with one-way sensitivity results of approximately 33,000,000/QALY.
CONCLUSION
The use of rhTSH is a cost-effective alternative to endogenous hypothyroid stimulation prior to radioiodine ablation for patients who have undergone thyroidectomy in Korea.
MeSH Terms
Figure
Cited by 1 articles
-
Triennial Report of
Endocrinology and Metabolism , 2015 to 2017
Eun-Jung Rhee, Hey Yeon Jang, Won-Young Lee
Endocrinol Metab. 2018;33(2):195-201. doi: 10.3803/EnM.2018.33.2.195.
Reference
-
1. Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W, et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006; 154:787–803.2. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–1214.3. Luster M, Felbinger R, Dietlein M, Reiners C. Thyroid hormone withdrawal in patients with differentiated thyroid carcinoma: a one hundred thirty-patient pilot survey on consequences of hypothyroidism and a pharmacoeconomic comparison to recombinant thyrotropin administration. Thyroid. 2005; 15:1147–1155.4. Dow KH, Ferrell BR, Anello C. Quality-of-life changes in patients with thyroid cancer after withdrawal of thyroid hormone therapy. Thyroid. 1997; 7:613–619.5. Rubic M, Kuna SK, Tesic V, Samardzic T, Despot M, Huic D. The most common factors influencing on quality of life of thyroid cancer patients after thyroid hormone withdrawal. Psychiatr Danub. 2014; 26:Suppl 3. 520–527.6. Lee J, Yun MJ, Nam KH, Chung WY, Soh EY, Park CS. Quality of life and effectiveness comparisons of thyroxine withdrawal, triiodothyronine withdrawal, and recombinant thyroid-stimulating hormone administration for low-dose radioiodine remnant ablation of differentiated thyroid carcinoma. Thyroid. 2010; 20:173–179.7. Borget I, Corone C, Nocaudie M, Allyn M, Iacobelli S, Schlumberger M, et al. Sick leave for follow-up control in thyroid cancer patients: comparison between stimulation with Thyrogen and thyroid hormone withdrawal. Eur J Endocrinol. 2007; 156:531–538.8. Schlumberger M, Catargi B, Borget I, Deandreis D, Zerdoud S, Bridji B, et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. 2012; 366:1663–1673.9. Mallick U, Harmer C, Yap B, Wadsley J, Clarke S, Moss L, et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med. 2012; 366:1674–1685.10. Pacini F, Ladenson PW, Schlumberger M, Driedger A, Luster M, Kloos RT, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. J Clin Endocrinol Metab. 2006; 91:926–932.11. Mernagh P, Suebwongpat A, Silverberg J, Weston A. Cost-effectiveness of using recombinant human thyroid-stimulating hormone before radioiodine ablation for thyroid cancer: the Canadian perspective. Value Health. 2010; 13:180–187.12. Schroeder PR, Haugen BR, Pacini F, Reiners C, Schlumberger M, Sherman SI, et al. A comparison of short-term changes in health-related quality of life in thyroid carcinoma patients undergoing diagnostic evaluation with recombinant human thyrotropin compared with thyroid hormone withdrawal. J Clin Endocrinol Metab. 2006; 91:878–884.13. Borget I, Remy H, Chevalier J, Ricard M, Allyn M, Schlumberger M, et al. Length and cost of hospital stay of radioiodine ablation in thyroid cancer patients: comparison between preparation with thyroid hormone withdrawal and thyrogen. Eur J Nucl Med Mol Imaging. 2008; 35:1457–1463.14. Health Insurance Review & Assessment Service. Korea reimbursement system [Internet]. Seoul: HIRA;c2013. cited 2015 Oct 22. Available from: http://www.hira.or.kr/rf/medicine/getStandardList.do?pgmid=HIRAA030035040000.15. Botella-Carretero JI, Galan JM, Caballero C, Sancho J, Escobar-Morreale HF. Quality of life and psychometric functionality in patients with differentiated thyroid carcinoma. Endocr Relat Cancer. 2003; 10:601–610.16. Brazier J, Usherwood T, Harper R, Thomas K. Deriving a preference-based single index from the UK SF-36 Health Survey. J Clin Epidemiol. 1998; 51:1115–1128.17. Sanofi. THYR-008-00: a randomized, controlled, open-label, multi-national pilot study of thyroid remnant ablation comparing the safety and ablation rate following 131I administration using ThyrogenTM versus the safety and ablation rate following 131I administration in the hypothyroid state: final report 2 [Internet]. Cambridge: Genzyme Corporation;c2015. cited 2015 Oct 22. Available from: http://www.genzymeclinicalresearch.com/home/search_clinical_trial_results/thyrogen_study5.asp.18. Luster M, Sherman SI, Skarulis MC, Reynolds JR, Lassmann M, Hanscheid H, et al. Comparison of radioiodine biokinetics following the administration of recombinant human thyroid stimulating hormone and after thyroid hormone withdrawal in thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2003; 30:1371–1377.19. Menzel C, Kranert WT, Dabert N, Diehl M, Fietz T, Hamscho N, et al. rhTSH stimulation before radioiodine therapy in thyroid cancer reduces the effective half-life of (131)I. J Nucl Med. 2003; 44:1065–1068.20. Hanscheid H, Lassmann M, Luster M, Thomas SR, Pacini F, Ceccarelli C, et al. Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawal. J Nucl Med. 2006; 47:648–654.21. Mernagh P, Campbell S, Dietlein M, Luster M, Mazzaferri E, Weston AR. Cost-effectiveness of using recombinant human TSH prior to radioiodine ablation for thyroid cancer, compared with treating patients in a hypothyroid state: the German perspective. Eur J Endocrinol. 2006; 155:405–414.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Retrospective Review of the Effectiveness of Recombinant Human TSH-Aided Radioiodine Treatment of Differentiated Thyroid Carcinoma
- Clinical Factors Associated with Quality of Life in Patients with Thyroid Cancer
- The Effectiveness of Recombinant Human Thyroid-Stimulating Hormone versus Thyroid Hormone Withdrawal Prior to Radioiodine Remnant Ablation in Thyroid Cancer: A Meta-Analysis of Randomized Controlled Trials
- Recent Advances in Radioiodine Therapy for Thyroid Cancer
- Surgical Methods for Radioiodine Refractory Thyroid Cancer