J Korean Med Sci.
2014 Jun;29(6):811-817. 10.3346/jkms.2014.29.6.811.
The Effectiveness of Recombinant Human Thyroid-Stimulating Hormone versus Thyroid Hormone Withdrawal Prior to Radioiodine Remnant Ablation in Thyroid Cancer: A Meta-Analysis of Randomized Controlled Trials
- Affiliations
-
- 1Department of Nuclear Medicine, Seoul National University Hospital, Seoul, Korea. larrycheon@gmail.com
- 2Department of Nuclear Medicine, Pusan National University Hospital, Busan, Korea.
- 3Medical Research Institute, Pusan National University Hospital, Busan, Korea.
- 4Cancer Research Institute, Seoul National University Hospital, Seoul, Korea.
- 5WCU Graduate School of Concergence Science and Technology, Seoul National University College of Medicine, Seoul, Korea.
- 6University of California at Irvine, CA, USA.
- KMID: 1796944
- DOI: http://doi.org/10.3346/jkms.2014.29.6.811
Abstract
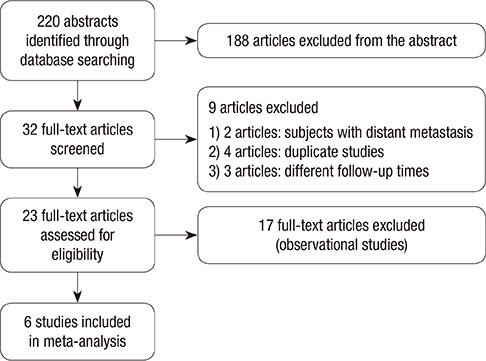
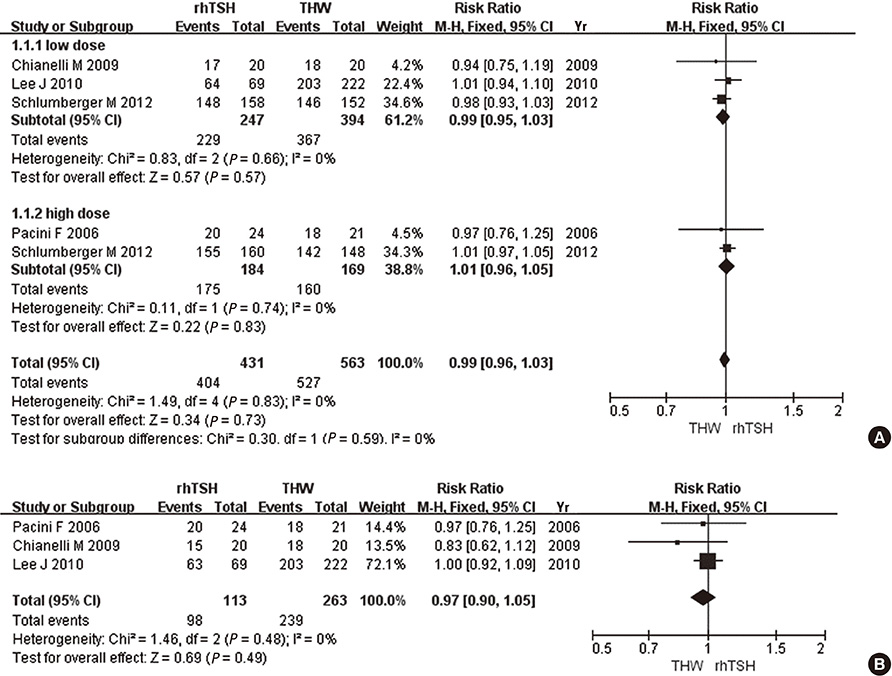
- We evaluated the efficacy of recombinant human thyroid-stimulating hormone (rhTSH) versus thyroid hormone withdrawal (THW) prior to radioiodine remnant ablation (RRA) in thyroid cancer. A systematic search of MEDLINE, EMBASE, the Cochrane Library, and SCOPUS was performed. Randomized controlled trials that compared ablation success between rhTSH and THW at 6 to 12 months following RRA were included in this study. Six trials with a total of 1,660 patients were included. When ablation success was defined as a thyroglobulin (Tg) cutoff of 1 ng/mL (risk ratio, 0.99; 95% confidence interval, 0.96-1.03) or a Tg cutoff of 1 ng/mL plus imaging modality (RR 0.97; 0.90-1.05), the results of rhTSH and THW were similar. There were no significant differences when ablation success was defined as a Tg cutoff of 2 ng/mL (RR 1.03; 0.95-1.11) or a Tg cutoff of 2 ng/mL plus imaging modality (RR 1.02; 0.95-1.09). When a negative 131I-whole body scan was used solely as the definition of ablation success, the effects of rhTSH and THW were not significantly different (RR 0.97; 0.93-1.02). Therefore, ablation success rates are comparable when RRA is prepared by either rhTSH or THW.
Keyword
MeSH Terms
-
Catheter Ablation
Clinical Trials as Topic
Databases, Factual
Humans
Iodine Radioisotopes/*therapeutic use
Radiopharmaceuticals/*therapeutic use
Recombinant Proteins/biosynthesis/genetics/therapeutic use
Risk
Thyroglobulin/analysis/metabolism
Thyroid Neoplasms/*drug therapy/ultrasonography
Thyrotropin/genetics/metabolism/*therapeutic use
Treatment Outcome
Whole Body Imaging
Iodine Radioisotopes
Radiopharmaceuticals
Recombinant Proteins
Thyroglobulin
Thyrotropin
Figure
Reference
-
1. Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012; 62:220–241.2. Lang BH, Law TT. The role of 18F-fluorodeoxyglucose positron emission tomography in thyroid neoplasms. Oncologist. 2011; 16:458–466.3. Robbins RJ, Schlumberger MJ. The evolving role of (131)I for the treatment of differentiated thyroid carcinoma. J Nucl Med. 2005; 46:28S–37S.4. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–1214.5. Pacini F, Ladenson PW, Schlumberger M, Driedger A, Luster M, Kloos RT, Sherman S, Haugen B, Corone C, Molinaro E, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. J Clin Endocrinol Metab. 2006; 91:926–932.6. Ma C, Xie J, Liu W, Wang G, Zuo S, Wang X, Wu F. Recombinant human thyrotropin (rhTSH) aided radioiodine treatment for residual or metastatic differentiated thyroid cancer. Cochrane Database Syst Rev. 2010; (11):CD008302.7. Chianelli M, Todino V, Graziano FM, Panunzi C, Pace D, Guglielmi R, Signore A, Papini E. Low-activity (2.0 GBq; 54 mCi) radioiodine post-surgical remnant ablation in thyroid cancer: comparison between hormone withdrawal and use of rhTSH in low-risk patients. Eur J Endocrinol. 2009; 160:431–436.8. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.9. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560.10. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–634.11. Taïeb D, Sebag F, Cherenko M, Baumstarck-Barrau K, Fortanier C, Farman-Ara B, De Micco C, Vaillant J, Thomas S, Conte-Devolx B, et al. Quality of life changes and clinical outcomes in thyroid cancer patients undergoing radioiodine remnant ablation (RRA) with recombinant human TSH (rhTSH): a randomized controlled study. Clin Endocrinol (Oxf). 2009; 71:115–123.12. Lee J, Yun MJ, Nam KH, Chung WY, Soh EY, Park CS. Quality of life and effectiveness comparisons of thyroxine withdrawal, triiodothyronine withdrawal, and recombinant thyroid-stimulating hormone administration for low-dose radioiodine remnant ablation of differentiated thyroid carcinoma. Thyroid. 2010; 20:173–179.13. Schlumberger M, Catargi B, Borget I, Deandreis D, Zerdoud S, Bridji B, Bardet S, Leenhardt L, Bastie D, Schvartz C, et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. 2012; 366:1663–1673.14. Mallick U, Harmer C, Yap B, Wadsley J, Clarke S, Moss L, Nicol A, Clark PM, Farnell K, McCready R, et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med. 2012; 366:1674–1685.15. Sawin CT, Becker DV. Radioiodine and the treatment of hyperthyroidism: the early history. Thyroid. 1997; 7:163–176.16. Chung JK, Youn HW, Kang JH, Lee HY, Kang KW. Sodium iodide symporter and the radioiodine treatment of thyroid carcinoma. Nucl Med Mol Imaging. 2010; 44:4–14.17. Hugo J, Robenshtok E, Grewal R, Larson S, Tuttle RM. Recombinant human thyroid stimulating hormone-assisted radioactive iodine remnant ablation in thyroid cancer patients at intermediate to high risk of recurrence. Thyroid. 2012; 22:1007–1015.18. Coliez R, Tubiana M, Dutreix J, Guelfi J. Results of examination of 85 cases of cancer of the thyroid with radioactive iodine. J Radiol Electrol Arch Electr Medicale. 1951; 32:881–895.19. Barbaro D, Boni G, Meucci G, Simi U, Lapi P, Orsini P, Pasquini C, Turco A, Mariani G. Recombinant human thyroid-stimulating hormone is effective for radioiodine ablation of post-surgical thyroid remnants. Nucl Med Commun. 2006; 27:627–632.20. Haugen BR, Pacini F, Reiners C, Schlumberger M, Ladenson PW, Sherman SI, Cooper DS, Graham KE, Braverman LE, Skarulis MC, et al. A comparison of recombinant human thyrotropin and thyroid hormone withdrawal for the detection of thyroid remnant or cancer. J Clin Endocrinol Metab. 1999; 84:3877–3885.21. Rosário PW, Borges MA, Purisch S. Preparation with recombinant human thyroid-stimulating hormone for thyroid remnant ablation with 131I is associated with lowered radiotoxicity. J Nucl Med. 2008; 49:1776–1782.22. Taïeb D, Sebag F, Farman-Ara B, Portal T, Baumstarck-Barrau K, Fortanier C, Bourrelly M, Mancini J, De Micco C, Auquier P, et al. Iodine biokinetics and radioiodine exposure after recombinant human thyrotropin-assisted remnant ablation in comparison with thyroid hormone withdrawal. J Clin Endocrinol Metab. 2010; 95:3283–3290.23. Frigo A, Dardano A, Danese E, Davì MV, Moghetti P, Colato C, Francia G, Bernardi F, Traino C, Monzani F, et al. Chromosome translocation frequency after radioiodine thyroid remnant ablation: a comparison between recombinant human thyrotropin stimulation and prolonged levothyroxine withdrawal. J Clin Endocrinol Metab. 2009; 94:3472–3476.24. Vallejo Casas JA, Mena Bares LM, Gálvez MA, Marlowe RJ, Latre Romero JM, Martínez-Paredes M. Treatment room length-of-stay and patient throughput with radioiodine thyroid remnant ablation in differentiated thyroid cancer: comparison of thyroid-stimulating hormone stimulation methods. Nucl Med Commun. 2011; 32:840–846.25. Borget I, Corone C, Nocaudie M, Allyn M, Iacobelli S, Schlumberger M, De Pouvourville G. Sick leave for follow-up control in thyroid cancer patients: comparison between stimulation with Thyrogen and thyroid hormone withdrawal. Eur J Endocrinol. 2007; 156:531–538.26. Mernagh P, Campbell S, Dietlein M, Luster M, Mazzaferri E, Weston AR. Cost-effectiveness of using recombinant human TSH prior to radioiodine ablation for thyroid cancer, compared with treating patients in a hypothyroid state: the German perspective. Eur J Endocrinol. 2006; 155:405–414.27. Dohán O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, Ginter CS, Carrasco N. The sodium/iodide Symporter (NIS): characterization, regulation, and medical significance. Endocr Rev. 2003; 24:48–77.28. Kogai T, Taki K, Brent GA. Enhancement of sodium/iodide symporter expression in thyroid and breast cancer. Endocr Relat Cancer. 2006; 13:797–826.29. Andros G, Wollman SH. Autoradiographic localization of radioiodide in the thyroid gland of the mouse. Am J Physiol. 1967; 213:198–208.30. Kogai T, Endo T, Saito T, Miyazaki A, Kawaguchi A, Onaya T. Regulation by thyroid-stimulating hormone of sodium/iodide symporter gene expression and protein levels in FRTL-5 cells. Endocrinology. 1997; 138:2227–2232.31. Mazzaferri EL. Thyroid remnant 131I ablation for papillary and follicular thyroid carcinoma. Thyroid. 1997; 7:265–271.32. Molinaro E, Giani C, Agate L, Biagini A, Pieruzzi L, Bianchi F, Brozzi F, Ceccarelli C, Viola D, Piaggi P, et al. Patients with differentiated thyroid cancer who underwent radioiodine thyroid remnant ablation with low-activity 131I after either recombinant human TSH or thyroid hormone therapy withdrawal showed the same outcome after a 10-year follow-up. J Clin Endocrinol Metab. 2013; 98:2693–2700.33. Ahn BC, Lee WK, Jeong SY, Lee SW, Lee J. Estimation of true serum thyroglobulin concentration using simultaneous measurement of serum antithyroglobulin antibody. Int J Endocrinol. 2013; 2013:210639.34. Yoo ID, Kim SH, Seo YY, Oh JK, O JH, Chung SK. The success rate of initial 131I ablation in differentiated thyroid cancer: comparison between less strict and very strict low iodine diets. Nucl Med Mol Imaging. 2012; 46:34–40.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Economic Evaluation of Recombinant Human Thyroid Stimulating Hormone Stimulation vs. Thyroid Hormone Withdrawal Prior to Radioiodine Ablation for Thyroid Cancer: The Korean Perspective
- Recent Advances in Radioiodine Therapy for Thyroid Cancer
- A Retrospective Review of the Effectiveness of Recombinant Human TSH-Aided Radioiodine Treatment of Differentiated Thyroid Carcinoma
- Low Dose versus High Dose Radioiodine Therapy
- Clinical Factors Associated with Quality of Life in Patients with Thyroid Cancer