Endocrinol Metab.
2010 Jun;25(2):103-109. 10.3803/EnM.2010.25.2.103.
Isolation of Density Enrichment Fraction of Adipose-Derived Stem Cells from Stromal Vascular Fraction by Gradient Centrifugation Method
- Affiliations
-
- 1Department of Food and Nutrition, Hanyang University, Seoul, Korea.
- 2Diabetes Research Institute, Kangbuk Samsung Medical Center, Seoul, Korea.
- 3Department of Internal Medicine, Kangbuk Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. cydoctor@chol.com
- 4Department of Endocrinology-Metabolism, College of Medicine, Kyunghee University, Seoul, Korea.
- KMID: 2169015
- DOI: http://doi.org/10.3803/EnM.2010.25.2.103
Abstract
- BACKGROUND
Adipose tissues include multipotent cells, the same as bone marrow-derived mesenchymal stem cells. The stromal vascular fractions (SVFs) from adipose tissues represent a heterogeneous cell population. The purpose of this study was to isolate and purify adipose-derived stem cells (ASCs) in SVFs by the density gradient method.
METHODS
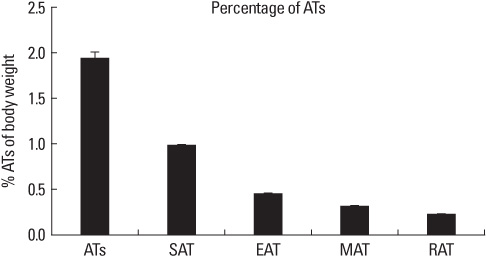
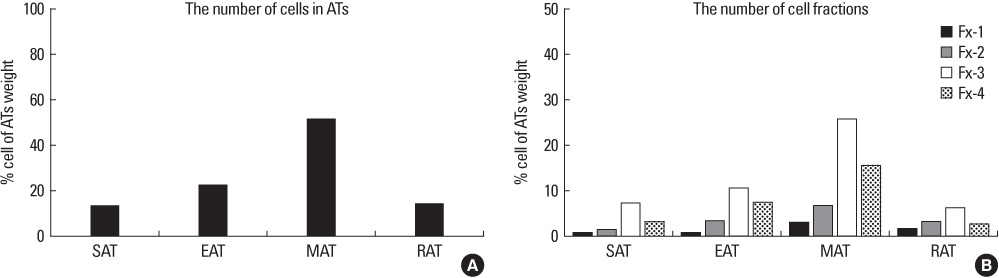
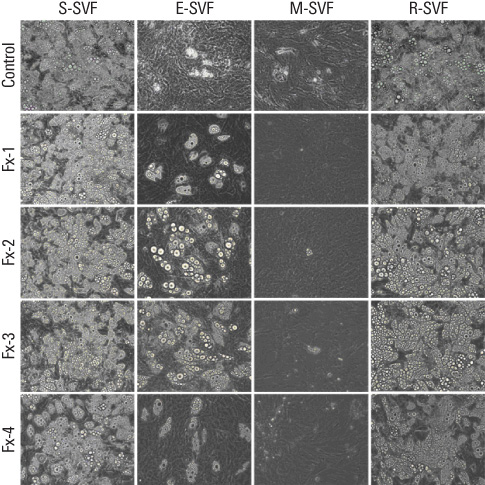
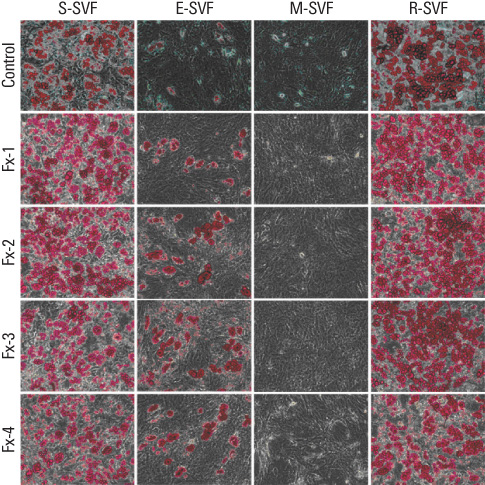
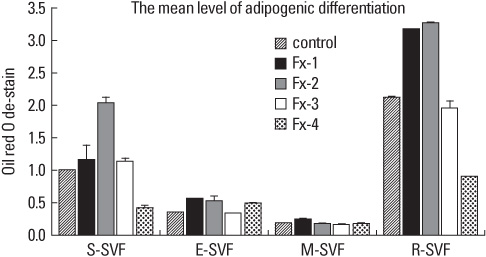
SVFs were extracted from the subcutaneous, epididymal, mesenteric and retroperitoneal adipose tissue of 8 weeks old male Sprague-Dawley rats (n = 15) and these were separated into 4 layers according to a Nycodenz gradient (Fx-1: < 11%, Fx-2: 11-13%, Fx-3: 13-19% and Fx-4: 19-30%). The post-confluent SVFs were cultured in adipogenic medium for 2 days, in insulin medium for 2 days and in 10% fetal bovine serum medium for 5 days. To observe lipid droplets in SVFs, we performed Oil Red O staining. RESLTS: The SVFs' cellular fractions (Fx-1, Fx-2, Fx-3 and Fx-4) were isolated by density gradient centrifugation from the adipose tissues of rats. The SVFs extracted to fraction 3 (Fx-3) had the most abundant cells compared to that of the other fractions. However fraction 1 (Fx-1) or 2 (Fx-2) had a superior ability to make lipid droplets. The adipogenic differentiation of Fx-1 or 2 was higher than that of the unfractionated cells. The SVFs extracted from retroperitoneal adipose tissue had the highest efficiency for adipogenic differentiation, whereas the SVFs from mesenteric adipose tissue did not differentiate.
CONCLUSION
This density gradient fractionated method leads to efficient isolation and purification of cells with the characteristics of ASCs.
Keyword
MeSH Terms
Figure
Reference
-
1. Jun YJ. Recent Development trend and prospects of adipose-derived stem cells on nerve regeneration. Tissue Eng Regen Med. 2008. 5:51–56.2. Yang YI, Kim HI, Seo JY, Choi MY. Adult stem cells as cell therapeutics for angiogenesis. Tissue Eng Regen Med. 2007. 4:484–489.3. Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998. 279:1528–1530.4. Kuznetsov SA, Friedenstein AJ, Robey PG. Factors required for bone marrow stromal fibroblast colony formation in vitro. Br J Haematol. 1997. 97:561–570.5. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.6. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997. 276:71–74.7. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000. 61:364–370.8. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002. 418:41–49.9. De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003. 174:101–109.10. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002. 13:4279–4295.11. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001. 7:211–228.12. Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, Fraser JK, Hedrick MH. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005. 54:132–141.13. Grégoire F, Todoroff G, Hauser N, Remacle C. The stroma-vascular fraction of rat inguinal and epididymal adipose tissue and the adipoconversion of fat cell precursors in primary culture. Biol Cell. 1990. 69:215–222.14. Alpini G, Phillips JO, Vroman B, LaRusso NF. Recent advances in the isolation of liver cells. Hepatology. 1994. 20:494–514.15. Innes GK, Fuller BJ, Hobbs KE. Functional testing of hepatocytes following their recovery from cryopreservation. Cryobiology. 1988. 25:23–30.16. Munthe-Kaas AC, Seglen PO. The use of Metrizamide as a gradient medium for isopycnic separation of rat liver cells. FEBS Lett. 1974. 43:252–256.17. Dabeva MD, Hwang SG, Vasa SR, Hurston E, Novikoff PM, Hixson DC, Gupta S, Shafritz DA. Differentiation of pancreatic epithelial progenitor cells into hepatocytes following transplantation into rat liver. Proc Natl Acad Sci U S A. 1997. 94:7356–7361.18. Rickwood D, Ford T, Graham J. Nycodenz: a new nonionic iodinated gradient medium. Anal Biochem. 1982. 123:23–31.19. Ford TC, Rickwood D. Formation of isotonic Nycodenz gradients for cell separations. Anal Biochem. 1982. 124:293–298.20. Rodbell M. Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem. 1964. 239:375–380.21. Rodbell M. Metabolism of isolated fat cells. II. The similar effects of phospholipase C (Clostridium perfringens alpha toxin) and of insulin on glucose and amino acid metabolism. J Biol Chem. 1966. 241:130–139.22. Rodbell M, Jones AB. Metabolism of isolated fat cells. 3. The similar inhibitory action of phospholipase C (Clostridium perfringens alpha toxin) and of insulin on lipolysis stimulated by lipolytic hormones and theophylline. J Biol Chem. 1966. 241:140–142.23. Rodbell M. The metabolism of isolated fat cells. IV. Regulation of release of protein by lipolytic hormones and insulin. J Biol Chem. 1966. 241:3909–3917.24. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006. 8:315–317.25. Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007. 9:204.26. Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001. 189:54–63.27. Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006. 24:376–385.28. Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005. 23:412–423.29. Yañez R, Lamana ML, Garcia-Castro J, Colmenero I, Ramirez M, Bueren JA. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006. 24:2582–2591.30. Haniffa MA, Wang XN, Holtick U, Rae M, Isaacs JD, Dickinson AM, Hilkens CM, Collin MP. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol. 2007. 179:1595–1604.31. Planat-Benard V, Silvestre JS, Cousin B, André M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Pénicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004. 109:656–663.32. Miranville A, Heeschen C, Sengenès C, Curat CA, Busse R, Bouloumié A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004. 110:349–355.33. Gaben-Cogneville AM, Aron Y, Idriss G, Jahchan T, Pello JY, Swierczewski E. Differentiation under the control of insulin of rat preadipocytes in primary culture. Isolation of homogeneous cellular fractions by gradient centrifugation. Biochim Biophys Acta. 1983. 762:437–444.34. Safford KM, Hicok KC, Safford SD, Halvorsen YD, Wilkison WO, Gimble JM, Rice HE. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun. 2002. 294:371–379.35. Ashjian PH, Elbarbary AS, Edmonds B, DeUgarte D, Zhu M, Zuk PA, Lorenz HP, Benhaim P, Hedrick MH. In vitro differentiation of human processed lipoaspirate cells into early neural progenitors. Plast Reconstr Surg. 2003. 111:1922–1931.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Letter: Isolation of Density Enrichment Fraction of Adipose-Derived Stem Cells from Stromal Vascular Fraction by Gradient Centrifugation Method
- Response: Isolation of Density Enrichment Fraction of Adipose-Derived Stem Cells from Stromal Vascular Fraction by Gradient Centrifugation Method
- Brief Review of Adipose Derived Cells
- A Novel Hypothesis and Characterization to Isolate Microvascular Endothelial Cells Simultaneously with AdiposeDerived Stem Cells from the Human Adipose-Derived Stromal Vascular Fraction
- Separation of Human Epidermal Langerhans Cells by Density Gradient Centrifugation on a Colloidal Silica ( Percoll ) Gradient Method and Autologous , Allogeneic Mixed Skin Cell Leukocyte Culture Reactions