Anat Cell Biol.
2014 Mar;47(1):44-54. 10.5115/acb.2014.47.1.44.
Nerves and fasciae in and around the paracolpium or paravaginal tissue: an immunohistochemical study using elderly donated cadavers
- Affiliations
-
- 1Department of Urology, Kobe University Graduate School of Medicine, Kobe, Japan. hinata@med.kobe-u.ac.jp
- 2Department of Urology, Hiroshima University School of Medicine, Hiroshima, Japan.
- 3Division of Gynecology and Obstetrics, Ishikawa Prefectural Central Hospital, Kanazawa, Japan.
- 4Department of Gynecology and Obstetrics, Fukui University School of Medicine, Fukui, Japan.
- 5Division of Internal Medicine, Iwamizawa Kojin-kai Hospital, Iwamizawa, Japan.
- 6Department of Anatomy, Sapporo Medical University School of Medicine, Sapporo, Japan.
- KMID: 2168856
- DOI: http://doi.org/10.5115/acb.2014.47.1.44
Abstract
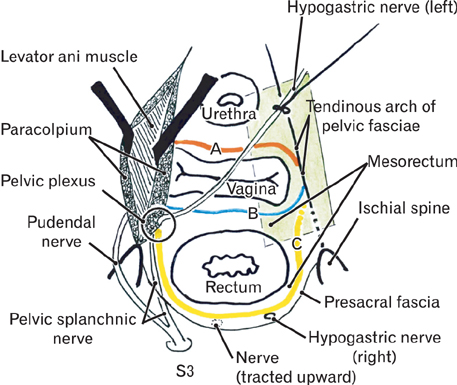
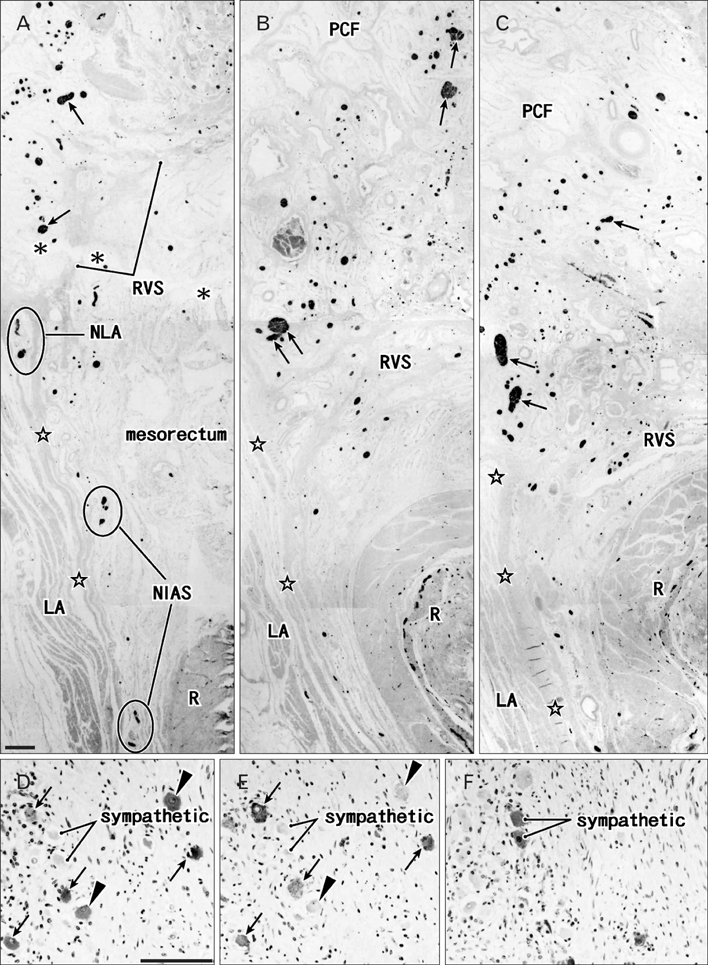
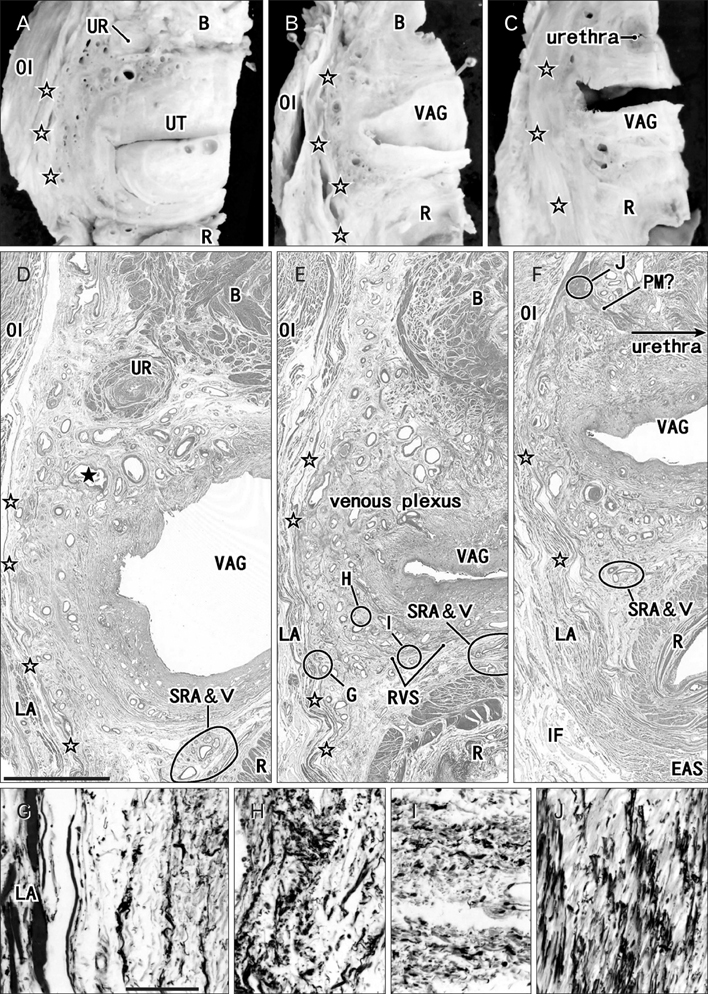
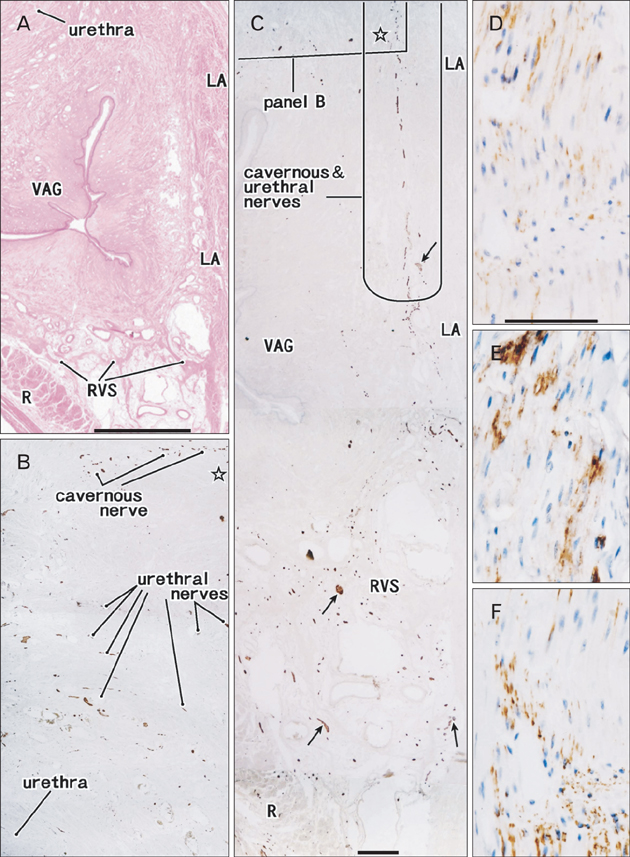
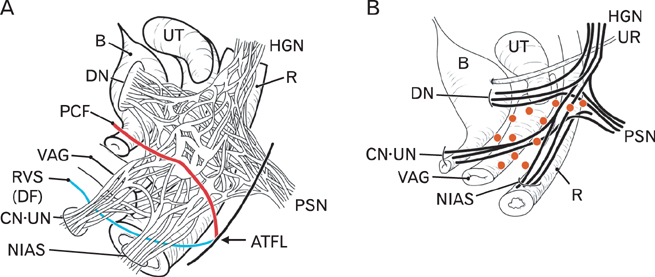
- The paracolpium or paravaginal tissue is surrounded by the vaginal wall, the pubocervical fascia and the rectovaginal septum (Denonvilliers' fascia). To clarify the configuration of nerves and fasciae in and around the paracolpium, we examined histological sections of 10 elderly cadavers. The paracolpium contained the distal part of the pelvic autonomic nerve plexus and its branches: the cavernous nerve, the nerves to the urethra and the nerves to the internal anal sphincter (NIAS). The NIAS ran postero-inferiorly along the superior fascia of the levator ani muscle to reach the longitudinal muscle layer of the rectum. In two nulliparous and one multiparous women, the pubocervical fascia and the rectovaginal septum were distinct and connected with the superior fascia of the levator at the tendinous arch of the pelvic fasciae. In these three cadavers, the pelvic plexus and its distal branches were distributed almost evenly in the paracolpium and sandwiched by the pubocervical and Denonvilliers' fasciae. By contrast, in five multiparous women, these nerves were divided into the anterosuperior group (bladder detrusor nerves) and the postero-inferior group (NIAS, cavernous and urethral nerves) by the well-developed venous plexus in combination with the fragmented or unclear fasciae. Although the small number of specimens was a major limitation of this study, we hypothesized that, in combination with destruction of the basic fascial architecture due to vaginal delivery and aging, the pelvic plexus is likely to change from a sheet-like configuration to several bundles.
Keyword
MeSH Terms
Figure
Reference
-
1. DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992; 166(6 Pt 1):1717–1724.2. Mostwin JL. Current concepts of female pelvic anatomy and physiology. Urol Clin North Am. 1991; 18:175–195.3. Mauroy B, Goullet E, Stefaniak X, Bonnal JL, Amara N. Tendinous arch of the pelvic fascia: application to the technique of paravaginal colposuspension. Surg Radiol Anat. 2000; 22:73–79.4. Pit MJ, De Ruiter MC, Lycklama AN, Marani E, Zwartendijk J. Anatomy of the arcus tendineus fasciae pelvis in females. Clin Anat. 2003; 16:131–137.5. Albright TS, Gehrich AP, Davis GD, Sabi FL, Buller JL. Arcus tendineus fascia pelvis: a further understanding. Am J Obstet Gynecol. 2005; 193(3 Pt 1):677–681.6. Zhai LD, Liu J, Li YS, Yuan W, He L. Denonvilliers' fascia in women and its relationship with the fascia propria of the rectum examined by successive slices of celloidin-embedded pelvic viscera. Dis Colon Rectum. 2009; 52:1564–1571.7. Zhang C, Ding ZH, Li GX, Yu J, Wang YN, Hu YF. Perirectal fascia and spaces: annular distribution pattern around the mesorectum. Dis Colon Rectum. 2010; 53:1315–1322.8. Donker PJ. A study of the myelinated fibres in the branches of the pelvic plexus. Neurourol Urodyn. 1986; 5:185–202.9. Ball TP Jr, Teichman JM, Sharkey FE, Rogenes VJ, Adrian EK Jr. Terminal nerve distribution to the urethra and bladder neck: considerations in the management of stress urinary incontinence. J Urol. 1997; 158(3 Pt 1):827–829.10. Kato M, Niikura H, Yaegashi N, Murakami G, Tatsumi H, Matsubara A. Histotopography of the female cavernous nerve: a study using donated fetuses and adult cadavers. Int Urogynecol J Pelvic Floor Dysfunct. 2008; 19:1687–1695.11. Hirata E, Koyama M, Murakami G, Ohtsuka A, Abe S, Ide Y, Fujiwara H, Kudo Y. Comparative histological study of levels 1-3 supportive tissues using pelvic floor semiserial sections from elderly nulliparous and multiparous women. J Obstet Gynaecol Res. 2011; 37:13–23.12. Yucel S, De Souza A Jr, Baskin LS. Neuroanatomy of the human female lower urogenital tract. J Urol. 2004; 172:191–195.13. Alsaid B, Moszkowicz D, Peschaud F, Bessede T, Zaitouna M, Karam I, Droupy S, Benoit G. Autonomic-somatic communications in the human pelvis: computer-assisted anatomic dissection in male and female fetuses. J Anat. 2011; 219:565–573.14. Hieda K, Cho KH, Arakawa T, Fujimiya M, Murakami G, Matsubara A. Nerves in the intersphincteric space of the human anal canal with special reference to their continuation to the enteric nerve plexus of the rectum. Clin Anat. 2013; 03. 20. [Epub]. http://dx.doi.org/10.1002/ca.22227.15. Hoyle CH, Stones RW, Robson T, Whitley K, Burnstock G. Innervation of vasculature and microvasculature of the human vagina by NOS and neuropeptide-containing nerves. J Anat. 1996; 188(Pt 3):633–644.16. Maas CP, DeRuiter MC, Kenter GG, Trimbos JB. The inferior hypogastric plexus in gynecologic surgery. J Gynecol Tech. 1999; 5:55–62.17. Baader B, Herrmann M. Topography of the pelvic autonomic nervous system and its potential impact on surgical intervention in the pelvis. Clin Anat. 2003; 16:119–130.18. Ercoli A, Delmas V, Fanfani F, Gadonneix P, Ceccaroni M, Fagotti A, Mancuso S, Scambia G. Terminologia Anatomica versus unofficial descriptions and nomenclature of the fasciae and ligaments of the female pelvis: a dissection-based comparative study. Am J Obstet Gynecol. 2005; 193:1565–1573.19. Yabuki Y, Sasaki H, Hatakeyama N, Murakami G. Discrepancies between classic anatomy and modern gynecologic surgery on pelvic connective tissue structure: harmonization of those concepts by collaborative cadaver dissection. Am J Obstet Gynecol. 2005; 193:7–15.20. Hollabaugh RS, Steiner MS, Dmochowski RR. Neuroanatomy of the female continence complex: clinical implications. Urology. 2001; 57:382–388.21. Tamakawa M, Murakami G, Takashima K, Kato T, Hareyama M. Fascial structures and autonomic nerves in the female pelvis: a study using macroscopic slices and their corresponding histology. Anat Sci Int. 2003; 78:228–242.22. Motohashi O, Suzuki M, Shida N, Umezawa K, Ohtoh T, Sakurai Y, Yoshimoto T. Subarachnoid haemorrhage induced proliferation of leptomeningeal cells and deposition of extracellular matrices in the arachnoid granulations and subarachnoid space. Immunhistochemical study. Acta Neurochir (Wien). 1995; 136:88–91.23. Hayashi T, Kumasaka T, Mitani K, Yao T, Suda K, Seyama K. Loss of heterozygosity on tuberous sclerosis complex genes in multifocal micronodular pneumocyte hyperplasia. Mod Pathol. 2010; 23:1251–1260.24. Kato M, Matsubara A, Murakami G, Abe S, Ide Y, Sato I, Usui T. Female perineal membrane: a study using pelvic floor semiserial sections from elderly nulliparous and multiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2008; 19:1663–1670.25. Niikura H, Jin ZW, Cho BH, Murakami G, Yaegashi N, Lee JK, Lee NH, Li CA. Human fetal anatomy of the coccygeal attachments of the levator ani muscle. Clin Anat. 2010; 23:566–574.26. Hinata N, Murakami G, Abe S, Honda M, Isoyama T, Sejima T, Takenaka A. Detailed histological investigation of the female urethra: application to radical cystectomy. J Urol. 2012; 187:451–456.27. Takenaka A, Soga H, Murakami G, Niikura H, Tatsumi H, Yaegashi N, Tanaka K, Fujisawa M. Understanding anatomy of "hilus" of detrusor nerves to avoid bladder dysfunction after pelvic surgery: demonstration using fetal and adult cadavers . Urology. 2009; 73:251–257.28. Leffler KS, Thompson JR, Cundiff GW, Buller JL, Burrows LJ, Schön Ybarra MA. Attachment of the rectovaginal septum to the pelvic sidewall. Am J Obstet Gynecol. 2001; 185:41–43.29. Nagata I, Murakami G, Suzuki D, Furuya K, Koyama M, Ohtsuka A. Histological features of the rectovaginal septum in elderly women and a proposal for posterior vaginal defect repair. Int Urogynecol J Pelvic Floor Dysfunct. 2007; 18:863–868.30. Hirata E, Fujiwara H, Hayashi S, Ohtsuka A, Abe S, Murakami G, Kudo Y. Intergender differences in histological architecture of the fascia pelvis parietalis: a cadaveric study. Clin Anat. 2011; 24:469–477.31. Takeyama M, Koyama M, Murakami G, Nagata I, Tomoe H, Furuya K. Nerve preservation in tension-free vaginal mesh procedures for pelvic organ prolapse: a cadaveric study using fresh and fixed cadavers. Int Urogynecol J Pelvic Floor Dysfunct. 2008; 19:559–566.32. Arakawa T, Murakami G, Nakajima F, Matsubara A, Ohtsuka A, Goto T, Teramoto T. Morphologies of the interfaces between the levator ani muscle and pelvic viscera, with special reference to muscle insertion into the anorectum in elderly Japanese. Anat Sci Int. 2004; 79:72–81.33. Kinugasa Y, Murakami G, Suzuki D, Sugihara K. Histological identification of fascial structures posterolateral to the rectum. Br J Surg. 2007; 94:620–626.34. Sato K, Sato T. The vascular and neuronal composition of the lateral ligament of the rectum and the rectosacral fascia. Surg Radiol Anat. 1991; 13:17–22.35. Lin M, Chen W, Huang L, Ni J, Yin L. The anatomy of lateral ligament of the rectum and its role in total mesorectal excision. World J Surg. 2010; 34:594–598.36. Açar HI, Kuzu MA. Important points for protection of the autonomic nerves during total mesorectal excision. Dis Colon Rectum. 2012; 55:907–912.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Topohistology of sympathetic and parasympathetic nerve fibers in branches of the pelvic plexus: an immunohistochemical study using donated elderly cadavers
- Anatomical and Radiological Study of the Vascular Distribution and Skin Territory for the Tensor Fasciae Latae Free Flap
- Laparoscopic Paravaginal repair for the Treatment of Symptomatic Cystocele
- Composite nerve fibers in the hypogastric and pelvic splanchnic nerves: an immunohistochemical study using elderly cadavers
- A Case Report of a Huge Epidermal Inclusion Cyst in the Paravaginal Space