Anat Cell Biol.
2015 Jun;48(2):114-123. 10.5115/acb.2015.48.2.114.
Composite nerve fibers in the hypogastric and pelvic splanchnic nerves: an immunohistochemical study using elderly cadavers
- Affiliations
-
- 1Faculty of Medical Science, Wonkwang University Graduate School, Iksan, Korea.
- 2Department of Neurology, Institute of Wonkwang Medical Science, Wonkwang University School of Medicine, Iksan, Korea. neurlogy@wonkwang.ac.kr
- 3Department of Urology, Hiroshima University School of Medicine, Hiroshima, Japan.
- 4Department of Anatomy, Chonbuk National University Medical School, Jeonju, Korea.
- 5Division of Internal Medicine, Iwamizawa Kojin-kai Hospital, Iwamizawa, Japan.
- 6Department of Anatomy, Tokyo Dental College, Tokyo, Japan.
- KMID: 1845279
- DOI: http://doi.org/10.5115/acb.2015.48.2.114
Abstract
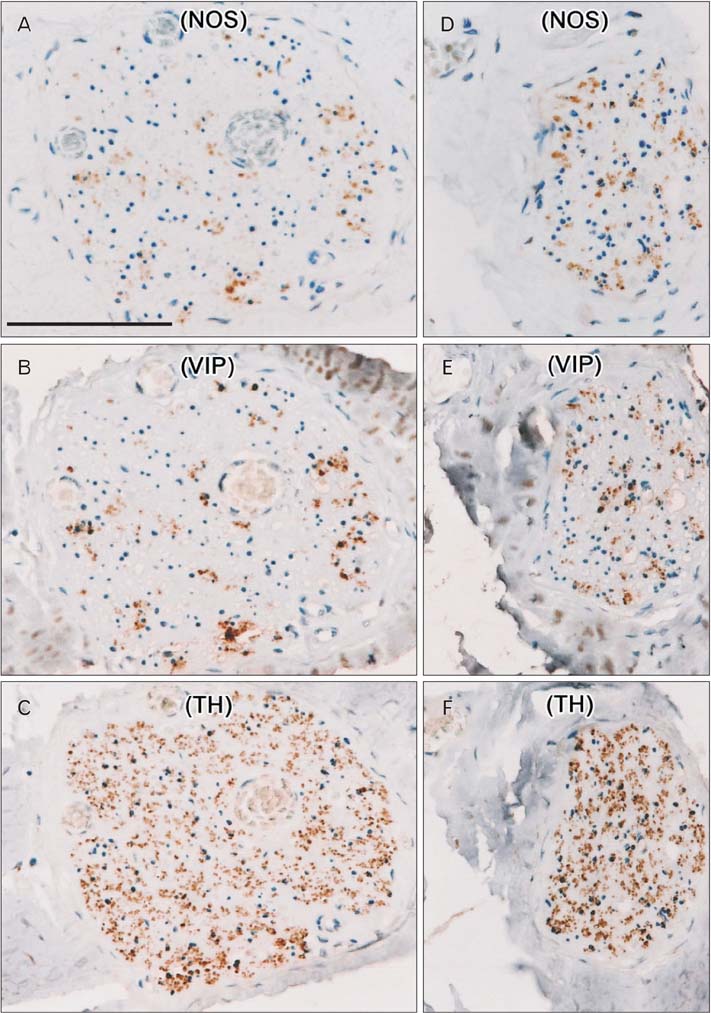
- To determine the proportion of nerve fibers in the hypogastric nerve (HGN) and pelvic splanchnic nerve (PSN), small tissue strips of the HGN and PSN from 12 donated elderly cadavers were examined histologically. Immunohistochemistry for neuronal nitric oxide synthase (NOS), vasoactive intestinal peptide (VIP), and tyrosine hydroxylase (TH) was performed. More than 70% of fibers per bundle in the HGN were positive for TH at the level of the sacral promontory. In addition, NOS- (negative) and/or VIP+ (positive) fibers were observed in small areas of each nerve bundle, although the proportion of each was usually less than 10%. In the PSN near the third sacral nerve root, the proportion of nerve fibers positive for NOS and/or VIP (or TH) was below 30%. In both the HGN and PSN, the number of VIP+ fibers was usually greater than that of NOS+ fibers, with frequent co-localization of NOS and VIP. More fibers in both nerves were positive for TH than for these other markers. In contrast to pelvic plexus branches, there were no differences in the proportions of NOS+ and VIP+ fibers between nerve bundles in each of the tissue strips. Thus, target-dependent sorting of nerve fibers was not apparent in the HGN at the level of the sacral promontory or in the PSN near the third sacral nerve root. The NOS+ and/or VIP+ fibers in the HGN were most likely ascending postganglionic fibers to the colon, while those in the PSN root may be preganglionic fibers from Onuf's nucleus.
Keyword
MeSH Terms
Figure
Reference
-
1. Maas CP, DeRuiter MC, Kenter GG, Trimbos JB. The inferior hypogastric plexus in gynecologic surgery. J Gynecol Tech. 1999; 5:55–62.2. Baader B, Herrmann M. Topography of the pelvic autonomic nervous system and its potential impact on surgical intervention in the pelvis. Clin Anat. 2003; 16:119–130.3. Mauroy B, Demondion X, Bizet B, Claret A, Mestdagh P, Hurt C. The female inferior hypogastric (= pelvic) plexus: anatomical and radiological description of the plexus and its afferences--applications to pelvic surgery. Surg Radiol Anat. 2007; 29:55–66.4. Hoyle CH, Stones RW, Robson T, Whitley K, Burnstock G. Innervation of vasculature and microvasculature of the human vagina by NOS and neuropeptide-containing nerves. J Anat. 1996; 188(Pt 3):633–644.5. Busacchi P, De Giorgio R, Santini D, Bellavia E, Perri T, Oliverio C, Paradisi R, Corinaldesi R, Flamigni C. A histological and immunohistochemical study of neuropeptide containing somatic nerves in the levator ani muscle of women with genitourinary prolapse. Acta Obstet Gynecol Scand. 1999; 78:2–5.6. Butler-Manuel SA, Buttery LD, A'Hern RP, Polak JM, Barton DP. Pelvic nerve plexus trauma at radical and simple hysterectomy: a quantitative study of nerve types in the uterine supporting ligaments. J Soc Gynecol Investig. 2002; 9:47–56.7. Butler-Manuel SA, Buttery LD, Polak JM, A'Hern R, Barton DP. Autonomic nerve trauma at radical hysterectomy: the nerve content and subtypes within the superficial and deep uterosacral ligaments. Reprod Sci. 2008; 15:91–96.8. Hisasue S, Hashimoto K, Kobayashi K, Takeuchi M, Kyoda Y, Sato S, Masumori N, Tsukamoto T. Baseline erectile function alters the cavernous nerve quantity and distribution around the prostate. J Urol. 2010; 184:2062–2067.9. Alsaid B, Bessede T, Karam I, Abd-Alsamad I, Uhl JF, Benoit G, Droupy S, Delmas V. Coexistence of adrenergic and cholinergic nerves in the inferior hypogastric plexus: anatomical and immunohistochemical study with 3D reconstruction in human male fetus. J Anat. 2009; 214:645–654.10. Moszkowicz D, Peschaud F, Bessede T, Benoit G, Alsaid B. Internal anal sphincter parasympathetic-nitrergic and sympathetic-adrenergic innervation: a 3-dimensional morphological and functional analysis. Dis Colon Rectum. 2012; 55:473–481.11. Takenaka A, Kawada M, Murakami G, Hisasue S, Tsukamoto T, Fujisawa M. Interindividual variation in distribution of extramural ganglion cells in the male pelvis: a semi-quantitative and immunohistochemical study concerning nerve-sparing pelvic surgery. Eur Urol. 2005; 48:46–52.12. Hinata N, Hieda K, Sasaki H, Murakami G, Abe S, Matsubara A, Miyake H, Fujisawa M. Topohistology of sympathetic and parasympathetic nerve fibers in branches of the pelvic plexus: an immunohistochemical study using donated elderly cadavers. Anat Cell Biol. 2014; 47:55–65.13. Yucel S, De Souza A Jr, Baskin LS. Neuroanatomy of the human female lower urogenital tract. J Urol. 2004; 172:191–195.14. Tamakawa M, Murakami G, Takashima K, Kato T, Hareyama M. Fascial structures and autonomic nerves in the female pelvis: a study using macroscopic slices and their corresponding histology. Anat Sci Int. 2003; 78:228–242.15. Maas K, Moriya Y, Kenter G, Trimbos B, van de Velde C. A plea for preservation of the pelvic autonomic nerves. Lancet. 1999; 354:772–773.16. Kinugasa Y, Murakami G, Suzuki D, Sugihara K. Histological identification of fascial structures posterolateral to the rectum. Br J Surg. 2007; 94:620–626.17. Kinugasa Y, Murakami G. The contents of lateral ligaments: is organized connective tissue present? Dis Colon Rectum. 2006; 49:1243–1244.18. Takeyama M, Koyama M, Murakami G, Nagata I, Tomoe H, Furuya K. Nerve preservation in tension-free vaginal mesh procedures for pelvic organ prolapse: a cadaveric study using fresh and fixed cadavers. Int Urogynecol J Pelvic Floor Dysfunct. 2008; 19:559–566.19. Akita K, Sakamoto H, Sato T. Origins and courses of the nervous branches to the male urethral sphincter. Surg Radiol Anat. 2003; 25:387–392.20. Hieda K, Cho KH, Arakawa T, Fujimiya M, Murakami G, Matsubara A. Nerves in the intersphincteric space of the human anal canal with special reference to their continuation to the enteric nerve plexus of the rectum. Clin Anat. 2013; 26:843–854.21. Bernard CE, Gibbons SJ, Gomez-Pinilla PJ, Lurken MS, Schmalz PF, Roeder JL, Linden D, Cima RR, Dozois EJ, Larson DW, Camilleri M, Zinsmeister AR, Pozo MJ, Hicks GA, Farrugia G. Effect of age on the enteric nervous system of the human colon. Neurogastroenterol Motil. 2009; 21:746–e46.22. Nakao T, Cho KH, Yamamoto M, Yamane S, Murakami G, Ide Y, Abe S. Site-dependent difference in the density of sympathetic nerve fibers in muscle-innervating nerves: a histologic study using human cadavers. Eur J Anat. 2012; 16:33–42.23. Kuntz A, Hoffman H, Schaeffer EM. Fiber components of the splanchnic nerves. Anat Rec. 1957; 128:139–146.24. Schnitzlein HN, Hoffman HH, Tucker CC, Quigley MB. The pelvic splanchnic nerves of the male Rheusus monkey. J Comp Neurol. 1960; 114:51–65.25. Imai K, Furuya K, Kawada M, Kinugasa Y, Omote K, Namiki A, Uchiyama E, Murakami G. Human pelvic extramural ganglion cells: a semiquantitative and immunohistochemical study. Surg Radiol Anat. 2006; 28:596–605.26. Hinata N, Hieda K, Sasaki H, Kurokawa T, Miyake H, Fujisawa M, Murakami G, Fujimiya M. Nerves and fasciae in and around the paracolpium or paravaginal tissue: an immunohistochemical study using elderly donated cadavers. Anat Cell Biol. 2014; 47:44–54.27. Gibson SJ, Polak JM, Katagiri T, Su H, Weller RO, Brownell DB, Holland S, Hughes JT, Kikuyama S, Ball J, Bloom SR, Steiner TJ, de Belleroche J, Clifford Rose F. A comparison of the distributions of eight peptides in spinal cord from normal controls and cases of motor neurone disease with special reference to Onuf's nucleus. Brain Res. 1988; 474:255–278.28. Pullen AH, Humphreys P, Baxter RG. Comparative analysis of nitric oxide synthase immunoreactivity in the sacral spinal cord of the cat, macaque and human. J Anat. 1997; 191(Pt 2):161–175.29. Hosaka F, Katori Y, Kawase T, Fujimiya M, Ohguro H. Site-dependent differences in density of sympathetic nerve fibers in muscle-innervating nerves of the human head and neck. Anat Sci Int. 2014; 89:101–111.30. Lindh B, Lundberg JM, Hökfelt T. NPY-, galanin-, VIP/PHI-, CGRP- and substance P-immunoreactive neuronal subpopulations in cat autonomic and sensory ganglia and their projections. Cell Tissue Res. 1989; 256:259–273.31. Roudenok V, Kuhnel W, Rogov Y, Nerovnja A. Developmental changes in vasoactive intestinal polypeptide immunoreactivity in the human paravertebral ganglia. Ann Anat. 1999; 181:561–565.32. Kiyokawa H, Katori Y, Cho KH, Murakami G, Kawase T, Cho BH. Reconsideration of the autonomic cranial ganglia: an immunohistochemical study of mid-term human fetuses. Anat Rec (Hoboken). 2012; 295:141–149.33. Maggi CA, Giuliani S, Santicioli P, Patacchini R, Said SI, Theodorsson E, Turini D, Barbanti G, Giachetti A, Meli A. Direct evidence for the involvement of vasoactive intestinal polypeptide in the motor response of the human isolated ileum to capsaicin. Eur J Pharmacol. 1990; 185:169–178.34. Jacobson ED, Berguer R, Pawlik WW, Hottenstein OD. Mesenteric purinergic and peptidergic vasodilators. In : Johnson LR, Alpers DH, Christensen J, Jacobson ED, Walsh JH, editors. Physiology of the Gastrointestinal Tract. New York: Raven Press;1994. p. 1647–1667.35. Kawatani M, Suzuki T, de Groat WC. Corticotropin releasing factor-like immunoreactivity in afferent projections to the sacral spinal cord of the cat. J Auton Nerv Syst. 1996; 61:218–226.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Topohistology of sympathetic and parasympathetic nerve fibers in branches of the pelvic plexus: an immunohistochemical study using donated elderly cadavers
- Superior Hypogastric Plexus Block for Malignant Pelvic Pain
- Nerve-sparing radical hysterectomy in the precision surgery for cervical cancer
- Penile erection evoked by autonomic nerve stimulation in rats
- Nerves and fasciae in and around the paracolpium or paravaginal tissue: an immunohistochemical study using elderly donated cadavers