Hanyang Med Rev.
2014 Nov;34(4):165-172. 10.7599/hmr.2014.34.4.165.
Hepatocyte Isolation, Culture, and Its Clinical Applications
- Affiliations
-
- 1Biomedical Research Institute, Lifeliver Co. Ltd., Yongin, Korea.
- 2Department of Surgery, Seoul National University College of Medicine, Seoul, Korea. kwleegs@gmail.com
- KMID: 2168348
- DOI: http://doi.org/10.7599/hmr.2014.34.4.165
Abstract
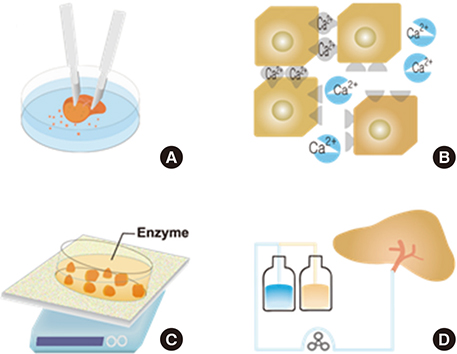
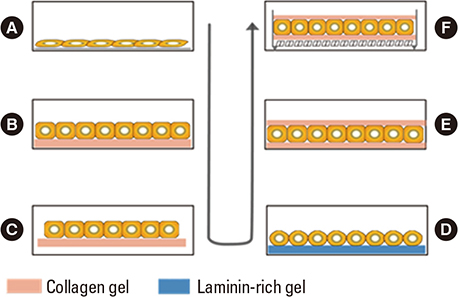
- Hepatocytes, parenchymal cells of the liver, are specially differentiated cells to perform most of the body metabolisms. Many clinicians are interested in utilizing hepatocytes as cell therapeutics. A great number of investigators have been harvesting hepatocytes using two-step portal vein perfusion method, in which Ca2+-free EDTA-containing buffer and Ca2+-enriched collagenase solution are pumped into liver in sequence. Among various attempts for long-term culture of hepatocytes, collagen gel sandwich configuration is recognized to be the most effective technique. In the biomedical field, hepatocytes have been used in three methods of applications. First is hepatocyte transplantation for the treatment of acute, chronic liver failure and metabolic diseases. Donated livers not suitable for organ transplantation are rare, which is the major human hepatocyte source. This shortage of human hepatocyte source is expected to be resolved by virtue of rapid progressing stem cell technologies. The second application is biological components of bioartificial liver (BAL) system for acute liver failure patients. Due to the lack of functional activity of clinically studied BAL systems and difficulty of establishing a manufacturing system for ready-to-use, additional research activities are stagnated. The third utilization of hepatocytes is in vitro drug screening studies such as drug metabolism, transport, biliary excretion, and toxicity tests. If cell therapeutic treatments using hepatocytes are clinically valuable to some types of liver diseases, the demand for liver transplantation would be significantly diminished.
MeSH Terms
-
Collagen
Collagenases
Drug Evaluation, Preclinical
End Stage Liver Disease
Hepatocytes*
Humans
Liver
Liver Diseases
Liver Failure, Acute
Liver Transplantation
Liver, Artificial
Metabolic Diseases
Metabolism
Organ Transplantation
Perfusion
Portal Vein
Research Personnel
Stem Cells
Toxicity Tests
Transplants
Virtues
Collagen
Collagenases
Figure
Cited by 1 articles
-
Cutting Edge Technologies in Organ Transplantation
Dongho Choi
Hanyang Med Rev. 2014;34(4):143-144. doi: 10.7599/hmr.2014.34.4.143.
Reference
-
1. Puviani AC, Ottolenghi C, Tassinari B, Pazzi P, Morsiani E. An update on high-yield hepatocyte isolation methods and on the potential clinical use of isolated liver cells. Comp Biochem Physiol A Mol Integr Physiol. 1998; 121:99–109.
Article2. Reid LM, Jefferson DM. Culturing hepatocytes and other differentiated cells. Hepatology. 1984; 4:548–559.
Article3. Dunn JC, Tompkins RG, Yarmush ML. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991; 7:237–245.
Article4. Clement B, Guguen-Guillouzo C, Campion JP, Glaise D, Bourel M, Guillouzo A. Long-term co-cultures of adult human hepatocytes with rat liver epithelial cells: modulation of albumin secretion and accumulation of extracellular material. Hepatology. 1984; 4:373–380.
Article5. Wang SR, Renaud G, Infante J, Catala D, Infante R. Isolation of rat hepatocytes with EDTA and their metabolic function in primary culture. In Vitro Cell Dev Biol. 1985; 21:526–530.
Article6. Howard RB, Christensen AK, Gibbs FA, Pesch LA. The enzymatic preparation of isolated intact parenchymal cells from rat liver. J Cell Biol. 1967; 35:675–684.
Article7. Berry MN, Friend DS. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969; 43:506–520.8. Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976; 13:29–83.9. Gerlach J, Brombacher J, Smith M, Neuhaus P. High yield hepatocyte isolation from pig livers for investigation of hybrid liver support systems: influence of collagenase concentration and body weight. J Surg Res. 1996; 62:85–89.
Article10. Gerlach JC, Mutig K, Sauer IM, Schrade P, Efimova E, Mieder T, et al. Use of primary human liver cells originating from discarded grafts in a bioreactor for liver support therapy and the prospects of culturing adult liver stem cells in bioreactors: a morphologic study. Transplantation. 2003; 76:781–786.
Article11. Hughes RD, Mitry RR, Dhawan A, Lehec SC, Girlanda R, Rela M, et al. Isolation of hepatocytes from livers from non-heart-beating donors for cell transplantation. Liver Transpl. 2006; 12:713–717.
Article12. Schlegel A, Rougemont O, Graf R, Clavien PA, Dutkowski P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J Hepatol. 2013; 58:278–286.
Article13. Sagias FG, Mitry RR, Hughes RD, Lehec SC, Patel AG, Rela M, et al. N-acetylcysteine improves the viability of human hepatocytes isolated from severely steatotic donor liver tissue. Cell Transplant. 2010; 19:1487–1492.
Article14. Vondran FW, Katenz E, Schwartlander R, Morgul MH, Raschzok N, Gong X, et al. Isolation of primary human hepatocytes after partial hepatectomy: criteria for identification of the most promising liver specimen. Artif Organs. 2008; 32:205–213.
Article15. Li AP, Colburn SM, Beck DJ. A simplified method for the culturing of primary adult rat and human hepatocytes as multicellular spheroids. In Vitro Cell Dev Biol. 1992; 28A:673–677.
Article16. Koide N, Sakaguchi K, Koide Y, Asano K, Kawaguchi M, Matsushima H, et al. Formation of multicellular spheroids composed of adult rat hepatocytes in dishes with positively charged surfaces and under other nonadherent environments. Exp Cell Res. 1990; 186:227–235.
Article17. Lee SA, No da Y, Kang E, Ju J, Kim DS, Lee SH. Spheroid-based three-dimensional liver-on-a-chip to investigate hepatocyte-hepatic stellate cell interactions and flow effects. Lab Chip. 2013; 13:3529–3537.
Article18. Bissell DM. Primary hepatocyte culture: substratum requirements and production of matrix components. Fed Proc. 1981; 40:2469–2473.19. Bissell DM, Arenson DM, Maher JJ, Roll FJ. Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver. J Clin Invest. 1987; 79:801–812.
Article20. De Bruyn T, Chatterjee S, Fattah S, Keemink J, Nicolai J, Augustijns P, et al. Sandwich-cultured hepatocytes: utility for in vitro exploration of hepatobiliary drug disposition and drug-induced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2013; 9:589–616.
Article21. De Bartolo L, Jarosch-Von Schweder G, Haverich A, Bader A. A novel full-scale flat membrane bioreactor utilizing porcine hepatocytes: cell viability and tissue-specific functions. Biotechnol Prog. 2000; 16:102–108.
Article22. Morin O, Normand C. Long-term maintenance of hepatocyte functional activity in co-culture: requirements for sinusoidal endothelial cells and dexamethasone. J Cell Physiol. 1986; 129:103–110.
Article23. Acikgoz A, Giri S, Cho MG, Bader A. Morphological and functional analysis of hepatocyte spheroids generated on poly-HEMA-treated surfaces under the influence of fetal calf serum and nonparenchymal cells. Biomolecules. 2013; 3:242–269.
Article24. Terry TL, Gallin WJ. Effects of fetal calf serum and disruption of cadherin function on the formation of bile canaliculi between hepatocytes. Exp Cell Res. 1994; 214:642–653.
Article25. Parzefall W, Erber E, Kainzbauer E, Schulte-Hermann R. Effects of insulin, glucagon and triiodothyronine on DNA synthesis in rat hepatocyte primary cultures induced by liver tumour promoters and EGF. Toxicol In Vitro. 1996; 10:183–193.
Article26. Matas AJ, Sutherland DE, Steffes MW, Mauer SM, Sowe A, Simmons RL, et al. Hepatocellular transplantation for metabolic deficiencies: decrease of plasms bilirubin in Gunn rats. Science. 1976; 192:892–894.
Article27. Fitzpatrick E, Mitry RR, Dhawan A. Human hepatocyte transplantation: state of the art. J Intern Med. 2009; 266:339–357.
Article28. Mito M, Kusano M, Kawaura Y. Hepatocyte transplantation in man. Transplant Proc. 1992; 24:3052–3053.
Article29. Grossman M, Rader DJ, Muller DW, Kolansky DM, Kozarsky K, Clark BJ 3rd, et al. A pilot study of ex vivo gene therapy for homozygous familial hypercholesterolaemia. Nat Med. 1995; 1:1148–1154.
Article30. Jorns C, Ellis EC, Nowak G, Fischler B, Nemeth A, Strom SC, et al. Hepatocyte transplantation for inherited metabolic diseases of the liver. J Intern Med. 2012; 272:201–223.
Article31. Mitry RR, Hughes RD, Dhawan A. Hepatocyte transplantation. J Clin Exp Hepatol. 2011; 1:109–114.
Article32. Filippi C, Dhawan A. Current status of human hepatocyte transplantation and its potential for Wilson's disease. Ann N Y Acad Sci. 2014; 1315:50–55.
Article33. Dhawan A, Puppi J, Hughes RD, Mitry RR. Human hepatocyte transplantation: current experience and future challenges. Nat Rev Gastroenterol Hepatol. 2010; 7:288–298.
Article34. Puppi J, Strom SC, Hughes RD, Bansal S, Castell JV, Dagher I, et al. Improving the techniques for human hepatocyte transplantation: report from a consensus meeting in London. Cell Transplant. 2012; 21:1–10.
Article35. Hughes RD, Mitry RR, Dhawan A. Current status of hepatocyte transplantation. Transplantation. 2012; 93:342–347.
Article36. van de Kerkhove MP, Hoekstra R, Chamuleau RA, van Gulik TM. Clinical application of bioartificial liver support systems. Ann Surg. 2004; 240:216–230.
Article37. Ellis AJ, Hughes RD, Wendon JA, Dunne J, Langley PG, Kelly JH, et al. Pilot-controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology. 1996; 24:1446–1451.
Article38. Millis JM, Cronin DC, Johnson R, Conjeevaram H, Conlin C, Trevino S, et al. Initial experience with the modified extracorporeal liver-assist device for patients with fulminant hepatic failure: system modifications and clinical impact. Transplantation. 2002; 74:1735–1746.
Article39. Demetriou AA, Brown RS Jr, Busuttil RW, Fair J, McGuire BM, Rosenthal P, et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004; 239:660–667. discussion 7-70.
Article40. Demetriou AA, Rozga J, Podesta L, Lepage E, Morsiani E, Moscioni AD, et al. Early clinical experience with a hybrid bioartificial liver. Scand J Gastroenterol Suppl. 1995; 208:111–117.
Article41. Rozga J. Liver support technology--an update. Xenotransplantation. 2006; 13:380–389.42. Sauer IM, Zeilinger K, Obermayer N, Pless G, Grunwald A, Pascher A, et al. Primary human liver cells as source for modular extracorporeal liver support--a preliminary report. Int J Artif Organs. 2002; 25:1001–1005.
Article43. Morsiani E, Pazzi P, Puviani AC, Brogli M, Valieri L, Gorini P, et al. Early experiences with a porcine hepatocyte-based bioartificial liver in acute hepatic failure patients. Int J Artif Organs. 2002; 25:192–202.
Article44. van de Kerkhove MP, Di Florio E, Scuderi V, Mancini A, Belli A, Bracco A, et al. Phase I clinical trial with the AMC-bioartificial liver. Int J Artif Organs. 2002; 25:950–959.
Article45. van de Kerkhove MP, Poyck PP, van Wijk AC, Galavotti D, Hoekstra R, van Gulik TM, et al. Assessment and improvement of liver specific function of the AMC-bioartificial liver. Int J Artif Organs. 2005; 28:617–630.
Article46. Park JK, Lee DH. Bioartificial liver systems: current status and future perspective. J Biosci Bioeng. 2005; 99:311–319.
Article47. Pan XP, Li LJ. Advances in cell sources of hepatocytes for bioartificial liver. Hepatobiliary Pancreat Dis Int. 2012; 11:594–605.
Article48. Hewitt NJ, Lechon MJ, Houston JB, Hallifax D, Brown HS, Maurel P, et al. Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab Rev. 2007; 39:159–234.
Article49. Salonen JS, Nyman L, Boobis AR, Edwards RJ, Watts P, Lake BG, et al. Comparative studies on the cytochrome p450-associated metabolism and interaction potential of selegiline between human liver-derived in vitro systems. Drug Metab Dispos. 2003; 31:1093–1102.
Article50. Ploss A, Khetani SR, Jones CT, Syder AJ, Trehan K, Gaysinskaya VA, et al. Persistent hepatitis C virus infection in microscale primary human hepatocyte cultures. Proc Natl Acad Sci U S A. 2010; 107:3141–3145.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Isolation and Culture of Pig Hepatocyte in Large Scale for the Application of Bioartificial Liver System
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- In vitro Culture of Hepatitis C Virus (HCV) Using Immortalized Hepatocyte
- Cryopreservation of Rat Hepatocytes for the Use of Primary Culture
- Differentiation Potential of Breast Milk-Derived Mesenchymal Stem Cells into Hepatocyte-Like Cells