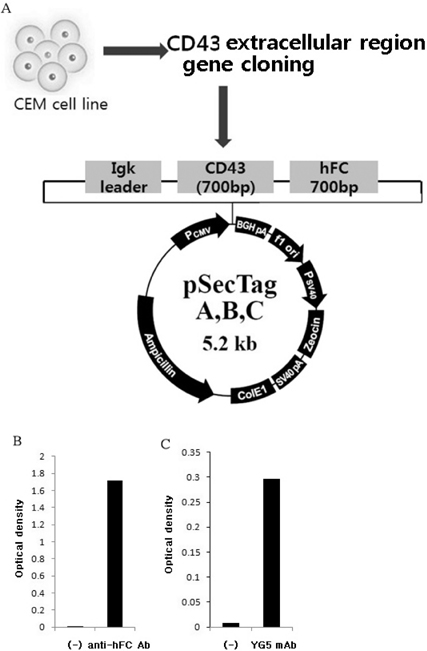
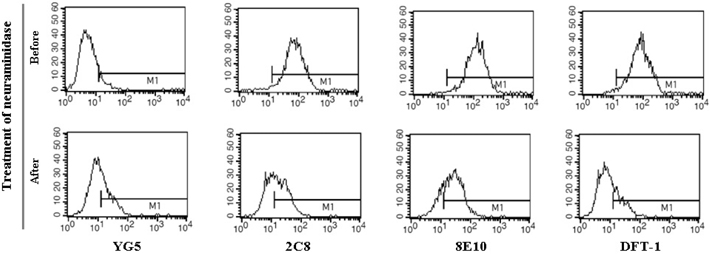
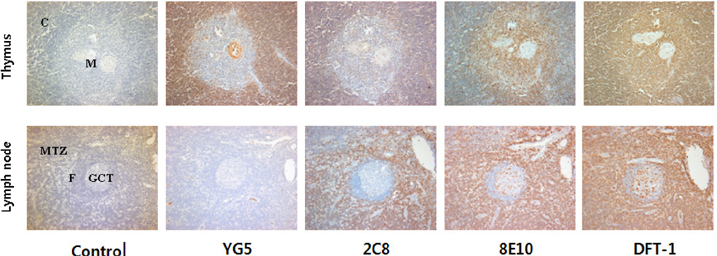
Immune Netw.
2014 Jun;14(3):164-170. 10.4110/in.2014.14.3.164.
Characterization of Two Novel mAbs Recognizing Different Epitopes on CD43
- Affiliations
-
- 1Department of Pathology, College of Medicine, Chungbuk National University, Cheongju 361-763, Korea. hgsong@chungbuk.ac.kr
- 2Research Institute, DiNonA Inc, Iksan 570-912, Korea.
- 3Graduate School of Health Science Convergence, College of Medicine, Chungbuk National University, Cheongju 361-763, Korea.
- 4MedClaris Inc, Seoul 100-210, Korea.
- KMID: 2168028
- DOI: http://doi.org/10.4110/in.2014.14.3.164
Abstract
- JL1, a specific epitope on CD43, is a potential biomarker for the diagnosis of acute leukemia. Although qualitative assays for detecting leukemia-specific CD43 exist, there is a need to develop quantitative assays for the same. Here, we developed two novel monoclonal antibodies (mAbs), 2C8 and 8E10, recognizing different epitopes on CD43. These clones are capable of pairing with YG5, another mAb against JL1 epitope, because they were selectively obtained using sandwich ELISA. Antigens recognized by 2C8 and 8E10 were confirmed as CD43 by western blotting using the CD43-hFC recombinant protein. When expression on various leukemic cell lines was investigated, 2C8 and 8E10 displayed a disparity in the distribution of the epitope. Enzyme assays revealed that these mAbs recognized a sialic acid-dependent epitope on CD43. Using normal thymus and lymph node paraffin-embedded tissues, we confirmed a difference in the epitopes recognized by the two mAbs that was predicted based on the maturity of the cells in the tissue. In summary, we developed and characterized two mAbs, 2C8 and 8E10, which can be used with YG5 in a sandwich ELISA for detecting leukemia-specific CD43.
Keyword
MeSH Terms
Figure
Reference
-
1. Remold-O'Donnell E. CD43 cluster report. In : Schlossman SF, Bounsell L, Gilks WR, Harlan J, Kishimoto T, morimoto T, Ritz J, Shaw S, Silverstein R, Springer T, Tedder T, Todd R, editors. Leukocyte typing V: white cell differentiation antigens. New York: Oxford University Press;1995. p. 1697–1701.2. Carlsson SR, Fukuda M. Isolation and characterization of leukosialin, a major sialoglycoprotein on human leukocytes. J Biol Chem. 1986; 261:12779–12786.
Article3. Shelley CS, Remold-ODonnell E, Davis AE, Bruns GA 3rd, Rosen FS, Carroll MC, Whitehead AS. Molecular characterization of sialophorin (CD43), the lymphocyte surface sialoglycoprotein defective in Wiskott-Aldrich syndrome. Proc Natl Acad Sci USA. 1989; 86:2819–2823.
Article4. Manjunath N, Mariangela C, Margarett A, Blair A. Negative regulation of T-cell adhesion and activation by CD43. Nature. 1995; 377:535–539.
Article5. Ardman B, Sikorski MA, Staunton DE. CD43 interferes with T-lymphocyte adhesion. Proc Natl Acad Sci USA. 1992; 89:5001–5005.
Article6. Seo W, Hermann JZ. CD43 processing and nuclear translocation of CD43 cytoplasmic tail are required for cell homeostasis. Blood. 2009; 114:3567–3577.
Article7. Rosenstein Y, Park JK, Hahn WC, Rosen FS, Bierer BE, Burakoff SJ. CD43, a molecule defective in Wiskott-Aldrich syndrome, binds ICAM-1. Nature. 1991; 354:233–235.
Article8. Shin YK, Choi EY, Kim SH, Chung J, Chung DH, Park WS, Jung KC, Kim HS, Park S, Kim HJ, Park MH, Min CK, Kim CC, Park SH. Expresion of Leukemia-Associated Antigen, JL1, in Bone Marrow and Thymus. Am J Pathol. 2001; 158:1473–1480.9. Park CS, Park SH. Diagnostic usefulness of monoclonal antibody for T lymphoblastic lymphoma/acute lymphoblastic leukemia-specific JL1 antigen in paraffin embedded tissue. Korean J Pathol. 1999; 33:1033–1038.10. Sahin U, Tureci O, Pfreundschuh M. Serological identification of human tumor antigens. Curr Opin Immunol. 1997; 9:709–716.
Article11. Chung JK, So Y, Hong MK, Choi SR, Jeong JM, Lee DS, Lee MC, Koh CS, Choi EY, Park SH. In vitro and in vivo properties of murine monoclonal antibody for a novel immature thymocyte-differentiated antigen, JL1. Nucl Med Biol. 1997; 24:433–437.
Article12. Park WS, Bae YM, Chung DH, Kim TJ, Choi EY, Chung JK, Lee MC, Park SY, Park SH. A cell surface molecule, JL1; a specific target for diagnosis and treatment of leukemias. Leukemia. 1998; 12:1583–1590.
Article13. Jeon YK, Min HS, Lee YJ, Kang BH, Kim EJ, Park HJ, Bae YM, Lee HG, Park WS, Song HG, Jung KC, Park SH. Targeting of developmentally regulated epitope of CD43 for the treatment of acute leukemia. Cancer Immunol Immunother. 2011; 60:1697–1706.
Article14. Frizzera G, Wu CD, Inghirami G. The usefulness of immunophenotypic and genotypic studies in the diagnosis and classification of hematopoietic and lymphoid neoplasma. Am J Clin Pathol. 1999; 111:S13–S39.15. Koníková E, Glasová M, Kusenda J, Babusíková O. Intracullular markers in acute myeloid leukemia diagnosis. Neoplasma. 1998; 45:282–291.16. Ohashi T, Mawatari K, Sato K, Tokeshi M, Kitamori T. A micro-ELISA system for the rapid and sensitive measurement of total and specific immunoglobulin E and clinical application to allergy diagnosis. Lab Chip. 2009; 9:991–995.
Article17. Cho WD. Detection of Lung Adenocarcinoma Using CD66c Specific Sandwich ELISA. Chungbook University;2012. Master Thesis.18. Shin MH. A novel monoclonal antibody 8F5 specific for CD66c of Lung Adenocarcinoma. Chungbook University;2009. Master Thesis.19. Scott AM, Welt S. Antibody-based immunological therapies. Curr Opin Immunol. 1997; 9:717–722.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characterization of Antigenic Sites on the Rinderpest Virus N Protein Uusing Monoclonal Antibodies
- Use of flow cytometry to develop and characterize a set of monoclonal antibodies specific for rabbit leukocyte differentiation molecules
- Human glutamate dehydrogenase is immunologically distinct from other mammalian orthologues
- Characterization and epitope mapping of two monoclonal antibodies against human CD99
- Expression of CD43 in Colorectal Adenocarcinom