Immune Netw.
2011 Oct;11(5):258-267. 10.4110/in.2011.11.5.258.
Gene Expression Profile of T-cell Receptors in the Synovium, Peripheral Blood, and Thymus during the Initial Phase of Collagen-induced Arthritis
- Affiliations
-
- 1Department of Medicine, Eulji Medi-Bio Research Institute, Eulji University, Daejeon 302-799, Korea. ssc@eulji.ac.kr
- 2Department of Physiology, School of Medicine, Eulji University, Daejeon 302-799, Korea.
- 3Genome Research Center for Immune Disorders, School of Medicine, Wonkwang University, Iksan 570-749, Korea.
- KMID: 2167998
- DOI: http://doi.org/10.4110/in.2011.11.5.258
Abstract
- BACKGROUND
Current management strategies attempt to diagnose rheumatoid arthritis (RA) at an early stage. Transcription profiling is applied in the search for biomarkers for detecting early-stage disease. Even though gene profiling has been reported using several animal models of RA, most studies were performed after the development of active arthritis, and conducted only on the peripheral blood and joint. Therefore, we investigated gene expression during the initial phase of collagen-induced arthritis (CIA) before the arthritic features developed in the thymus in addition to the peripheral blood and synovium.
METHODS
For gene expression analysis using cDNA microarray technology, samples of thymus, blood, and synovium were collected from CIA, rats immunized only with type II collagen (Cll), rats immunized only with adjuvant, and unimmunized rats on days 4 and 9 after the first immunization. Arrays were scanned with an Illumina bead array.
RESULTS
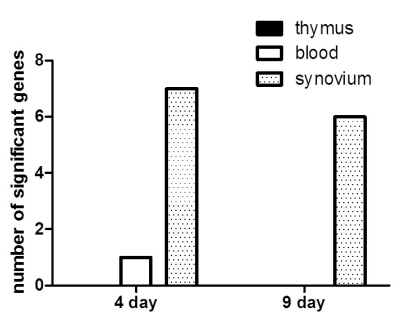
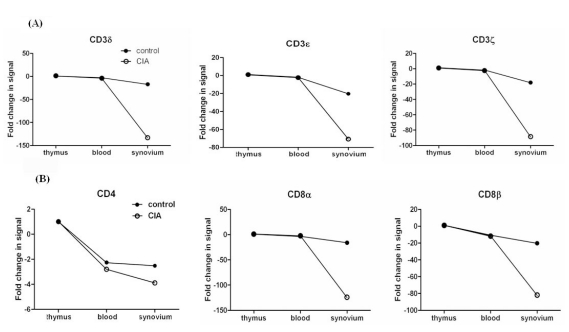
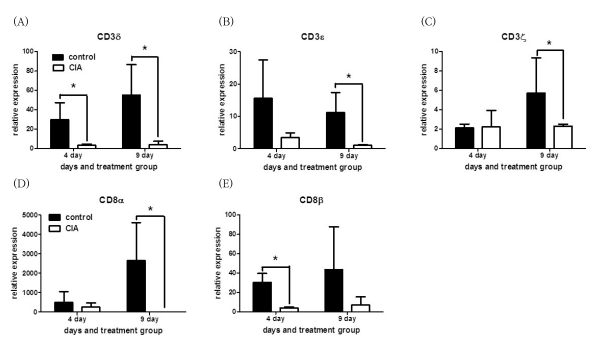
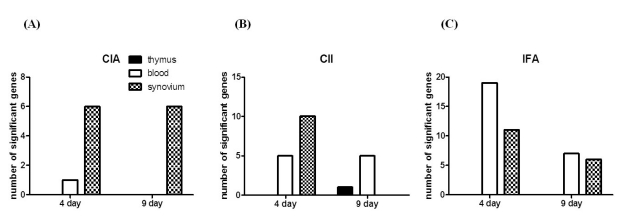
Of the 21,910 genes in the array, 1,243 genes were differentially expressed at least 2-fold change in various organs of CIA compared to controls. Among the 1,243 genes, 8 encode T-cell receptors (TCRs), including CD3zeta, CD3delta, CD3epsilon, CD8alpha, and CD8beta genes, which were down-regulated in CIA. The synovium was the organ in which the genes were differentially expressed between CIA and control group, and no difference were found in the thymus and blood. Further, we determined that the differential expression was affected by adjuvant more than Cll. The differential expression of genes as revealed by real-time RT-PCR, was in agreement with the microarray data.
CONCLUSION
This study provides evidence that the genes encoding TCRs including CD3zeta, CD3delta, CD3epsilon, CD8alpha, and CD8beta genes were down-regulated during the initial phase of CIA in the synovium of CIA. In addition, adjuvant played a greater role in the down-regulation of the CD3 complex compared to CII. Therefore, the down-regulation of TCR gene expression occurred dominantly by adjuvant could be involved in the pathogenesis of the early stage at CIA.
MeSH Terms
-
Animals
Antigens, CD3
Arthritis
Arthritis, Experimental
Arthritis, Rheumatoid
Biomarkers
Collagen Type II
Down-Regulation
Gene Expression
Genes, T-Cell Receptor
Immunization
Joints
Models, Animal
Oligonucleotide Array Sequence Analysis
Rats
Receptors, Antigen, T-Cell
Synovial Membrane
T-Lymphocytes
Thymus Gland
Transcriptome
Antigens, CD3
Collagen Type II
Receptors, Antigen, T-Cell
Figure
Reference
-
1. Firestein GS, Zvaifler NJ. Rheumatoid Arthritis: A Disease of Disordered Immunity. 1992. New York: Raven Press.2. Aho K, Heliövaara M, Maatela J, Tuomi T, Palosuo T. Rheumatoid factors antedating clinical rheumatoid arthritis. J Rheumatol. 1991; 18:1282–1284. PMID: 1757925.3. Cush JJ. Early rheumatoid arthritis - is there a window of opportunity? J Rheumatol Suppl. 2007; 80:1–7. PMID: 17985417.4. Durie FH, Fava RA, Noelle RJ. Collagen-induced arthritis as a model of rheumatoid arthritis. Clin Immunol Immunopathol. 1994; 73:11–18. PMID: 7923907.5. Brunsberg U, Gustafsson K, Jansson L, Michaëlsson E, Ahrlund-Richter L, Pettersson S, Mattsson R, Holmdahl R. Expression of a transgenic class II Ab gene confers susceptibility to collagen-induced arthritis. Eur J Immunol. 1994; 24:1698–1702. PMID: 8026530.6. Stuart JM, Townes AS, Kang AH. Nature and specificity of the immune response to collagen in type II collagen-induced arthritis in mice. J Clin Invest. 1982; 69:673–683. PMID: 6174550.
Article7. Wooley PH, Luthra HS, Stuart JM, David CS. Type II collagen-induced arthritis in mice. I. Major histocompatibility complex (I region) linkage and antibody correlates. J Exp Med. 1981; 154:688–700. PMID: 6792316.
Article8. Myers LK, Rosloniec EF, Cremer MA, Kang AH. Collagen-induced arthritis, an animal model of autoimmunity. Life Sci. 1997; 61:1861–1878. PMID: 9364191.
Article9. Thornton S, Sowders D, Aronow B, Witte DP, Brunner HI, Giannini EH, Hirsch R. DNA microarray analysis reveals novel gene expression profiles in collagen-induced arthritis. Clin Immunol. 2002; 105:155–168. PMID: 12482389.
Article10. Choy EH. Selective modulation of T-cell co-stimulation: a novel mode of action for the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2009; 27:510–518. PMID: 19604448.11. Fournier C. Where do T cells stand in rheumatoid arthritis? Joint Bone Spine. 2005; 72:527–532. PMID: 16087382.
Article12. Mandik-Nayak L, Allen PM. Initiation of an autoimmune response: insights from a transgenic model of rheumatoid arthritis. Immunol Res. 2005; 32:5–13. PMID: 16106055.
Article13. Sakaguchi N, Takahashi T, Hata H, Nomura T, Tagami T, Yamazaki S, Sakihama T, Matsutani T, Negishi I, Nakatsuru S, Sakaguchi S. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003; 426:454–460. PMID: 14647385.
Article14. Nandakumar KS, Svensson L, Holmdahl R. Collagen type II-specific monoclonal antibody-induced arthritis in mice: description of the disease and the influence of age, sex, and genes. Am J Pathol. 2003; 163:1827–1837. PMID: 14578183.15. Miceli MC, Parnes JR. The roles of CD4 and CD8 in T cell activation. Semin Immunol. 1991; 3:133–141. PMID: 1909592.16. Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996; 14:397–440. PMID: 8717520.
Article17. Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, Smolen J, Emery P, Harriman G, Feldmann M, Lipsky P. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999; 354:1932–1939. PMID: 10622295.18. Moreland LW, Baumgartner SW, Schiff MH, Tindall EA, Fleischmann RM, Weaver AL, Ettlinger RE, Cohen S, Koopman WJ, Mohler K, Widmer MB, Blosch CM. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997; 337:141–147. PMID: 9219699.
Article19. Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, Smolen JS, Weisman M, Emery P, Feldmann M, Harriman GR, Maini RN. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000; 343:1594–1602. PMID: 11096166.20. Booth G, Newham P, Barlow R, Raines S, Zheng B, Han S. Gene expression profiles at different stages of collagen-induced arthritis. Autoimmunity. 2008; 41:512–521. PMID: 18608173.
Article21. Calin A, Elswood J, Klouda PT. Destructive arthritis, rheumatoid factor, and HLA-DR4. Susceptibility versus severity, a case-control study. Arthritis Rheum. 1989; 32:1221–1225. PMID: 2487036.
Article22. Winchester R. The molecular basis of susceptibility to rheumatoid arthritis. Adv Immunol. 1994; 56:389–466. PMID: 7521116.
Article23. Van Boxel JA, Paget SA. Predominantly T-cell infiltrate in rheumatoid synovial membranes. N Engl J Med. 1975; 293:517–520. PMID: 168488.
Article24. Kraan MC, Smeets TJ, van Loon MJ, Breedveld FC, Dijkmans BA, Tak PP. Differential effects of leflunomide and methotrexate on cytokine production in rheumatoid arthritis. Ann Rheum Dis. 2004; 63:1056–1061. PMID: 15115713.
Article25. Berthelot JM, le Goff B, Maugars Y. Thymic Hassall's corpuscles, regulatory T-cells, and rheumatoid arthritis. Semin Arthritis Rheum. 2010; 39:347–355. PMID: 18973928.
Article26. Call ME, Wucherpfennig KW. Molecular mechanisms for the assembly of the T cell receptor-CD3 complex. Mol Immunol. 2004; 40:1295–1305. PMID: 15072848.
Article27. Kuhns MS, Davis MM, Garcia KC. Deconstructing the form and function of the TCR/CD3 complex. Immunity. 2006; 24:133–139. PMID: 16473826.
Article28. Kiessling R, Kono K, Petersson M, Wasserman K. Immunosuppression in human tumor-host interaction: role of cytokines and alterations in signal-transducing molecules. Springer Semin Immunopathol. 1996; 18:227–242. PMID: 8908702.
Article29. Finke JH, Zea AH, Stanley J, Longo DL, Mizoguchi H, Tubbs RR, Wiltrout RH, OShea JJ, Kudoh S, Klein E, Bukowski RM, Ochoa AC. Loss of T-cell receptor zeta chain and p56lck in T-cells infiltrating human renal cell carcinoma. Cancer Res. 1993; 53:5613–5616. PMID: 8242613.30. Maurice MM, Lankester AC, Bezemer AC, Geertsma MF, Tak PP, Breedveld FC, van Lier RA, Verweij CL. Defective TCR-mediated signaling in synovial T cells in rheumatoid arthritis. J Immunol. 1997; 159:2973–2978. PMID: 9300721.
Article31. Matsuda M, Ulfgren AK, Lenkei R, Petersson M, Ochoa AC, Lindblad S, Andersson P, Klareskog L, Kiessling R. Decreased expression of signal-transducing CD3 zeta chains in T cells from the joints and peripheral blood of rheumatoid arthritis patients. Scand J Immunol. 1998; 47:254–262. PMID: 9519864.32. Trimble LA, Lieberman J. Circulating CD8 T lymphocytes in human immunodeficiency virus-infected individuals have impaired function and downmodulate CD3 zeta, the signaling chain of the T-cell receptor complex. Blood. 1998; 91:585–594. PMID: 9427713.33. Berg L, Rönnelid J, Klareskog L, Bucht A. Down-regulation of the T cell receptor CD3 zeta chain in rheumatoid arthritis (RA) and its influence on T cell responsiveness. Clin Exp Immunol. 2000; 120:174–182. PMID: 10759780.34. Tartour E, Latour S, Mathiot C, Thiounn N, Mosseri V, Joyeux I, D'Enghien CD, Lee R, Debre B, Fridman WH. Variable expression of CD3-zeta chain in tumor-infiltrating lymphocytes (TIL) derived from renal-cell carcinoma: relationship with TIL phenotype and function. Int J Cancer. 1995; 63:205–212. PMID: 7591205.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In Vivo Transfection of Rabbit Synovium using the Particle Bombardment Method
- Lessons for the pathogenesis of rheumatoid arthritis acquired from experimental animal models
- Down-regulation of Type I Collagen Gene Expression in Human Skin Fibroblasts by G1 Cell Cycle Arrest
- The Correlation between the Expression of CD99 and the Cell Cycle
- Screening of Genes Regulating TNF-alpha-mediated Synovial Hyperplasia in Rheumatoid Arthritis