Cancer Res Treat.
2013 Jun;45(2):134-144.
RASSF1A Suppresses Cell Migration through Inactivation of HDAC6 and Increase of Acetylated alpha-Tubulin
- Affiliations
-
- 1Brain Korea 21 Project for Biomedical Science, Korea University College of Medicine, Seoul, Korea. yhk0215@korea.ac.kr
- 2Genomic Research Center for Lung and Breast/Ovarian Cancers, Korea University College of Medicine, Seoul, Korea.
- 3Division of Oncology/Hematology, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
The RAS association domain family protein 1 (RASSF1) has been implicated in a tumor-suppressive function through the induction of acetylated alpha-tubulin and modulation of cell migration. However, the mechanisms of how RASSF1A is associated with acetylation of alpha-tubulin for controlling cell migration have not yet been elucidated. In this study, we found that RASSF1A regulated cell migration through the regulation of histon deacetylase 6 (HDAC6), which functions as a tubulin deacetylase.
MATERIALS AND METHODS
The cell migration was assessed using wound-healing and transwell assays. The role of RASSF1A on cell migration was examined by immunofluorescence staining, HDAC activity assay and western blot analysis.
RESULTS
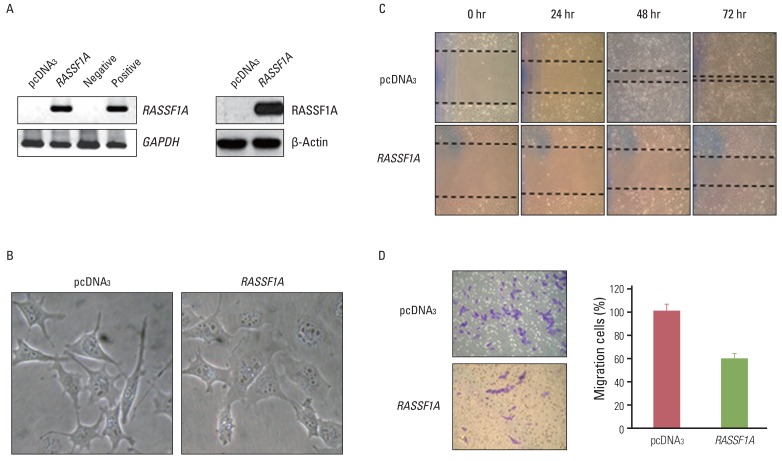
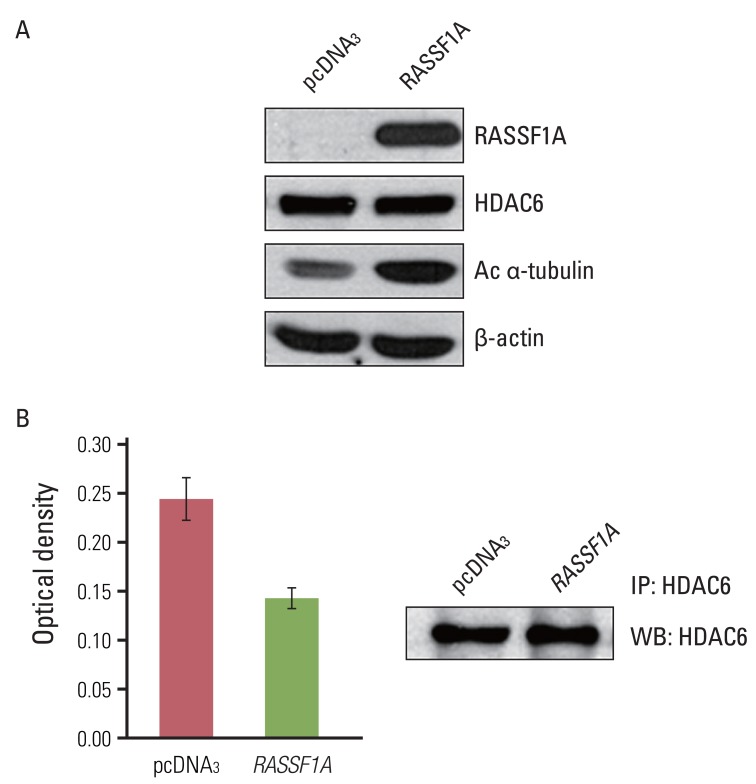
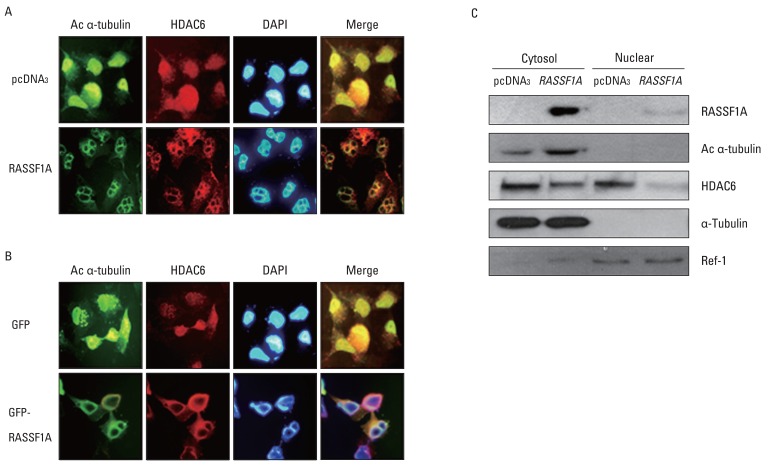
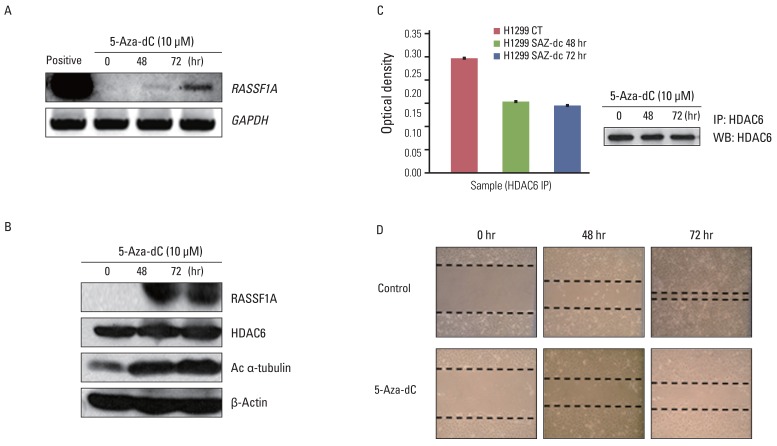
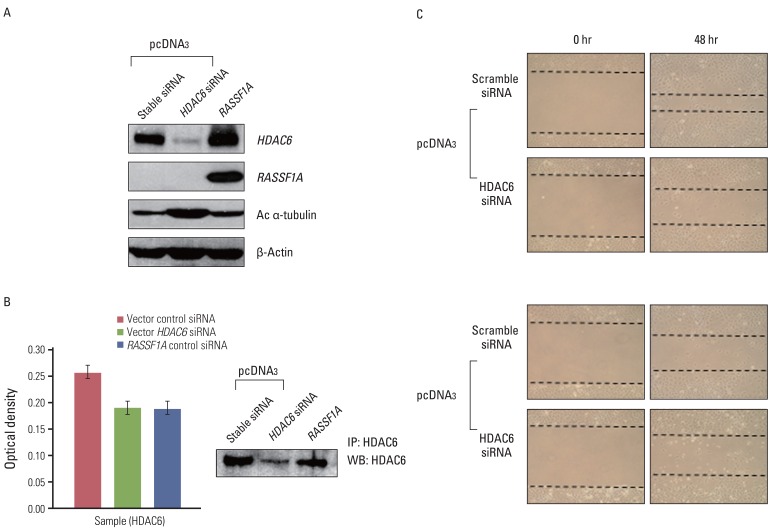
Cell migration was inhibited and cell morphology was changed in RASSF1A-transfected H1299 cells, compared with controls, whereas HDAC6 protein expression was not changed by RASSF1A transfection in these cells. However, RASSF1A inhibited deacetylating activity of HDAC6 protein and induced acetylated alpha-tubulin expression. Furthermore, acetylated alpha-tubulin and HDAC6 protein were co-localized in the cytoplasm in RASSF1A-transfected H1299 cells. Conversely, when the endogenous RASSF1A expression in HeLa cells was blocked with RASSF1A siRNA treatment, acetylated alpha-tubulin was co-localized with HDAC6 protein throughout the whole cells, including the nucleus, compared with scramble siRNA-treated HeLa cells. The restoration of RASSF1A by 5-Aza-dC treatment also induced acetylated alpha-tubulin through inhibition of HDAC6 activity that finally resulted in suppressing cell migration in H1299 cells. To further confirm the role of HDAC6 in RASSF1A-mediated cell migration, the HDAC6 expression in H1299 cells was suppressed by using HDAC6 siRNA, and cell motility was found to be decreased through enhanced acetylated alpha-tubulin.
CONCLUSION
The results of this study suggest that the inactivation of HDAC6 by RASSF1A regulates cell migration through increased acetylated alpha-tubulin protein.
Keyword
MeSH Terms
Figure
Reference
-
1. Lerman MI, Minna JD. The International Lung Cancer Chromosome 3p213 Tumor Suppressor Gene Consortium. The 630-kb lung cancer homozygous deletion region on human chromosome 3p21.3: identification and evaluation of the resident candidate tumor suppressor genes. Cancer Res. 2000; 60:6116–6133. PMID: 11085536.2. Dammann R, Li C, Yoon JH, Chin PL, Bates S, Pfeifer GP. Epigenetic inactivation of a RAS association domain family protein from the lung tumour suppressor locus 3p21.3. Nat Genet. 2000; 25:315–319. PMID: 10888881.
Article3. Pfeifer GP, Dammann R. Methylation of the tumor suppressor gene RASSF1A in human tumors. Biochemistry (Mosc). 2005; 70:576–583. PMID: 15948711.
Article4. Aoyama Y, Avruch J, Zhang XF. Nore1 inhibits tumor cell growth independent of Ras or the MST1/2 kinases. Oncogene. 2004; 23:3426–3433. PMID: 15007383.
Article5. Avruch J, Praskova M, Ortiz-Vega S, Liu M, Zhang XF. Nore1 and RASSF1 regulation of cell proliferation and of the MST1/2 kinases. Methods Enzymol. 2006; 407:290–310. PMID: 16757333.
Article6. Song MS, Song SJ, Ayad NG, Chang JS, Lee JH, Hong HK, et al. The tumour suppressor RASSF1A regulates mitosis by inhibiting the APC-Cdc20 complex. Nat Cell Biol. 2004; 6:129–137. PMID: 14743218.
Article7. Ahmed-Choudhury J, Agathanggelou A, Fenton SL, Ricketts C, Clark GJ, Maher ER, et al. Transcriptional regulation of cyclin A2 by RASSF1A through the enhanced binding of p120E4F to the cyclin A2 promoter. Cancer Res. 2005; 65:2690–2697. PMID: 15805267.
Article8. Fenton SL, Dallol A, Agathanggelou A, Hesson L, Ahmed-Choudhury J, Baksh S, et al. Identification of the E1A-regulated transcription factor p120 E4F as an interacting partner of the RASSF1A candidate tumor suppressor gene. Cancer Res. 2004; 64:102–107. PMID: 14729613.9. Geli J, Kiss N, Lanner F, Foukakis T, Natalishvili N, Larsson O, et al. The Ras effectors NORE1A and RASSF1A are frequently inactivated in pheochromocytoma and abdominal paraganglioma. Endocr Relat Cancer. 2007; 14:125–134. PMID: 17395981.
Article10. Oh HJ, Lee KK, Song SJ, Jin MS, Song MS, Lee JH, et al. Role of the tumor suppressor RASSF1A in Mst1-mediated apoptosis. Cancer Res. 2006; 66:2562–2569. PMID: 16510573.
Article11. Vos MD, Dallol A, Eckfeld K, Allen NP, Donninger H, Hesson LB, et al. The RASSF1A tumor suppressor activates Bax via MOAP-1. J Biol Chem. 2006; 281:4557–4563. PMID: 16344548.
Article12. Vos MD, Martinez A, Elam C, Dallol A, Taylor BJ, Latif F, et al. A role for the RASSF1A tumor suppressor in the regulation of tubulin polymerization and genomic stability. Cancer Res. 2004; 64:4244–4250. PMID: 15205337.
Article13. Dallol A, Agathanggelou A, Fenton SL, Ahmed-Choudhury J, Hesson L, Vos MD, et al. RASSF1A interacts with microtubule-associated proteins and modulates microtubule dynamics. Cancer Res. 2004; 64:4112–4116. PMID: 15205320.
Article14. El-Kalla M, Onyskiw C, Baksh S. Functional importance of RASSF1A microtubule localization and polymorphisms. Oncogene. 2010; 29:5729–5740. PMID: 20697344.
Article15. Dallol A, Agathanggelou A, Tommasi S, Pfeifer GP, Maher ER, Latif F. Involvement of the RASSF1A tumor suppressor gene in controlling cell migration. Cancer Res. 2005; 65:7653–7659. PMID: 16140931.
Article16. Verdel A, Curtet S, Brocard MP, Rousseaux S, Lemercier C, Yoshida M, et al. Active maintenance of mHDA2/mHDAC6 histone-deacetylase in the cytoplasm. Curr Biol. 2000; 10:747–749. PMID: 10873806.
Article17. Smith DS, Niethammer M, Ayala R, Zhou Y, Gambello MJ, Wynshaw-Boris A, et al. Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian Lis1. Nat Cell Biol. 2000; 2:767–775. PMID: 11056530.
Article18. Zhang Y, Li N, Caron C, Matthias G, Hess D, Khochbin S, et al. HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J. 2003; 22:1168–1179. PMID: 12606581.
Article19. Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002; 417:455–458. PMID: 12024216.
Article20. Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci U S A. 2003; 100:4389–4394. PMID: 12677000.
Article21. Agathanggelou A, Cooper WN, Latif F. Role of the Ras-association domain family 1 tumor suppressor gene in human cancers. Cancer Res. 2005; 65:3497–3508. PMID: 15867337.
Article22. Rong R, Jin W, Zhang J, Sheikh MS, Huang Y. Tumor suppressor RASSF1A is a microtubule-binding protein that stabilizes microtubules and induces G2/M arrest. Oncogene. 2004; 23:8216–8230. PMID: 15378022.
Article23. Liu L, Tommasi S, Lee DH, Dammann R, Pfeifer GP. Control of microtubule stability by the RASSF1A tumor suppressor. Oncogene. 2003; 22:8125–8136. PMID: 14603253.
Article24. Agathanggelou A, Bieche I, Ahmed-Choudhury J, Nicke B, Dammann R, Baksh S, et al. Identification of novel gene expression targets for the Ras association domain family 1 (RASSF1A) tumor suppressor gene in non-small cell lung cancer and neuroblastoma. Cancer Res. 2003; 63:5344–5351. PMID: 14500366.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Isoproterenol increases histone deacetylase 6 expression and cell migration by inhibiting ERK signaling via PKA and Epac pathways in human lung cancer cells
- ACY-241, a histone deacetylase 6 inhibitor, suppresses the epithelial–mesenchymal transition in lung cancer cells by downregulating hypoxia-inducible factor-1 alpha
- Shortening of primary cilia length is associated with urine concentration in the kidneys
- Potential Prognostic Value of Histone Deacetylase 6 and Acetylated Heat-Shock Protein 90 in Early-Stage Breast Cancer
- Evaluation of alpha-Tubulin as an Antigenic and Molecular Probe to Detect Giardia lamblia