Allergy Asthma Immunol Res.
2016 Jan;8(1):69-78. 10.4168/aair.2016.8.1.69.
Alternative Method for Primary Nasal Epithelial Cell Culture Using Intranasal Brushing and Feasibility for the Study of Epithelial Functions in Allergic Rhinitis
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Soonchunhyang University College of Medicine, Cheonan Hospital, Cheonan, Korea.
- 2BK21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Otorhinolaryngology, Yonsei University College of Medicine, Seoul, Korea.
- 4Airway Mucus Institute, Yonsei University College of Medicine, Seoul, Korea. hyunjerry@snu.ac.kr
- 5Research Center for Natural Human Defense System, Yonsei University College of Medicine, Seoul, Korea.
- 6Department of Otorhinolaryngology, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2166655
- DOI: http://doi.org/10.4168/aair.2016.8.1.69
Abstract
- PURPOSE
Although differentiated normal human nasal epithelial (NHNE) cells can be used to study the role of human nasal epithelium, there is a need for effective culture models of nasal epithelium in sinonasal disease status, including allergic rhinitis (AR). We aimed to examine the feasibility of intranasal brushing for culture of nasal epithelial cells in AR patients and to verify the hypothesis that allergic nasal epithelial (ARNE) cells differ in histologic and physiologic characteristics.
METHODS
We established a system for isolating (via intranasal brushing) and culturing (with air-liquid interface, ALI) nasal epithelial cells from healthy volunteers (n=8) and AR patients (n=8). We used this system to compare the histologic findings and physiologic characteristics of NHNE and ARNE.
RESULTS
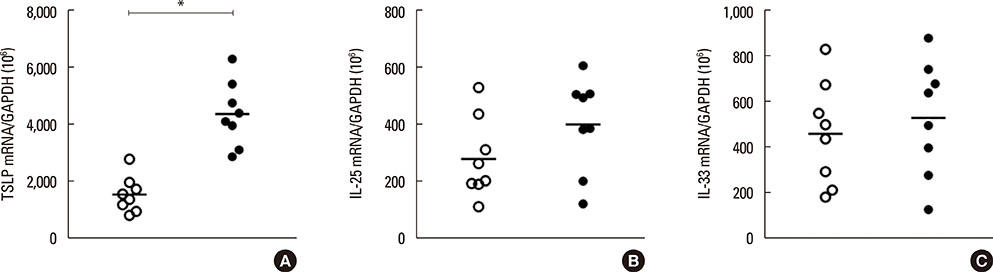
The histology results showed that fully differentiated ALI culture was obtained at least 14 days after confluence and that both ciliated and secretory cells were well differentiated in ALI culture using nasal brushing. The histology results of ARNE culture were significantly different from NHNE. The number of ciliated cells was lower, and secretory cells were more dominant in ARNE cell culture compared to NHNE cells. We also observed, by electron microscopy, loose tight junctions and short cilia in cultured ARNE cells. In addition, the mRNA level of TSLP which was one of the epithelial-derived allergic cytokines was significantly higher, and the expressions of genes involved in ciliogenesis were lower in cultured ARNE cells without allergen stimulation.
CONCLUSIONS
Our findings suggest that ALI culture of ARNE cells using intranasal brushing may be an alternative method for epithelial cell culture in AR patients and that cultured ARNE cells will be useful for in vitro studies of the mechanisms at play during AR because they maintain unique allergic characteristics.
MeSH Terms
Figure
Reference
-
1. Emerman JT, Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977; 13:316–328.2. Bridges MA, Walker DC, Harris RA, Wilson BR, Davidson AG. Cultured human nasal epithelial multicellular spheroids: polar cystlike model tissues. Biochem Cell Biol. 1991; 69:102–108.3. Ostrowski LE, Nettesheim P. Inhibition of ciliated cell differentiation by fluid submersion. Exp Lung Res. 1995; 21:957–970.4. Yoon JH, Gray T, Guzman K, Koo JS, Nettesheim P. Regulation of the secretory phenotype of human airway epithelium by retinoic acid, triiodothyronine, and extracellular matrix. Am J Respir Cell Mol Biol. 1997; 16:724–731.5. Gray TE, Guzman K, Davis CW, Abdullah LH, Nettesheim P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol. 1996; 14:104–112.6. Yoon JH, Kim KS, Kim SS, Lee JG, Park IY. Secretory differentiation of serially passaged normal human nasal epithelial cells by retinoic acid: expression of mucin and lysozyme. Ann Otol Rhinol Laryngol. 2000; 109:594–601.7. Hussain R, Hugosson S, Roomans GM. Isolation and culture of primary human nasal epithelial cells from anesthetized nasal epithelia. Acta Otolaryngol. 2014; 134:296–299.8. Bridges MA, Walker DC, Davidson AG. Cystic fibrosis and control nasal epithelial cells harvested by a brushing procedure. In Vitro Cell Dev Biol. 1991; 27A:684–686.9. Li CW, Zhang KK, Li TY, Lin ZB, Li YY, Curotto de Lafaille MA, et al. Expression profiles of regulatory and helper T-cell-associated genes in nasal polyposis. Allergy. 2012; 67:732–740.10. Yan Y, Gordon WM, Wang DY. Nasal epithelial repair and remodeling in physical injury, infection, and inflammatory diseases. Curr Opin Otolaryngol Head Neck Surg. 2013; 21:263–270.11. Li C, Shi L, Yan Y, Gordon BR, Gordon WM, Wang DY. Gene expression signatures: a new approach to understanding the pathophysiology of chronic rhinosinusitis. Curr Allergy Asthma Rep. 2013; 13:209–217.12. Cookson W. The immunogenetics of asthma and eczema: a new focus on the epithelium. Nat Rev Immunol. 2004; 4:978–988.13. Holgate ST. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol Rev. 2011; 242:205–219.14. Saglani S, Payne DN, Zhu J, Wang Z, Nicholson AG, Bush A, et al. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med. 2007; 176:858–864.15. Parker J, Sarlang S, Thavagnanam S, Williamson G, O'donoghue D, Villenave R, et al. A 3-D well-differentiated model of pediatric bronchial epithelium demonstrates unstimulated morphological differences between asthmatic and nonasthmatic cells. Pediatr Res. 2010; 67:17–22.16. Hackett TL, Warner SM, Stefanowicz D, Shaheen F, Pechkovsky DV, Murray LA, et al. Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-beta1. Am J Respir Crit Care Med. 2009; 180:122–133.17. Ogita-Nakanishi H, Nabe T, Mizutani N, Fujii M, Kohno S. Absence of nasal blockage in a Japanese cedar pollen-induced allergic rhinitis model mouse. Allergol Int. 2009; 58:171–178.18. Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008; 226:172–190.19. Kamekura R, Kojima T, Takano K, Go M, Sawada N, Himi T. The role of IL-33 and its receptor ST2 in human nasal epithelium with allergic rhinitis. Clin Exp Allergy. 2012; 42:218–228.20. Yu X, Ng CP, Habacher H, Roy S. Foxj1 transcription factors are master regulators of the motile ciliogenic program. Nat Genet. 2008; 40:1445–1453.21. Cheng DT, Ma C, Niewoehner J, Dahl M, Tsai A, Zhang J, et al. Thymic stromal lymphopoietin receptor blockade reduces allergic inflammation in a cynomolgus monkey model of asthma. J Allergy Clin Immunol. 2013; 132:455–462.22. Kouzaki H, Tojima I, Kita H, Shimizu T. Transcription of interleukin-25 and extracellular release of the protein is regulated by allergen proteases in airway epithelial cells. Am J Respir Cell Mol Biol. 2013; 49:741–750.23. Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci U S A. 2007; 104:914–919.24. Bartemes KR, Kita H. Dynamic role of epithelium-derived cytokines in asthma. Clin Immunol. 2012; 143:222–235.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In Vitro Culture of Human Nasal Epithelial Cells by Monolayer Culture of Dissociated Cells
- Expression of Neurokinin 1 Receptor in Allergic Rhinitis
- Primary Nasal Epithelial Cells From Allergic and Non-allergic Individuals Show Comparable Barrier Function
- Ciliary Activity of Upper Airway Epithelial Cells of Rats with Experimentally Induced Allergic Rhinitis
- Intranasal Phototherapy in the Patients with Perennial Allergic Rhinitis