Cancer Res Treat.
2005 Dec;37(6):349-353.
A Multi-Center, Phase II Clinical Trial of Padexol(TM) (Paclitaxel) and Cisplatin for Patients Suffering with Advanced Gastric Cancer
- Affiliations
-
- 1Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea. lkhee@medical.yu.ac.kr
- 2Department of Internal Medicine, Keimyung University College of Medicine, Daegu, Korea.
- 3Department of Internal Medicine, Fatima Hospital, Korea.
- 4Department of Internal Medicine, Dongguk University College of Medicine, Gyeongju, Korea.
- 5Department of Internal Medicine, Kyungpook National University Hospital, Daegu, Korea.
Abstract
- PURPOSE
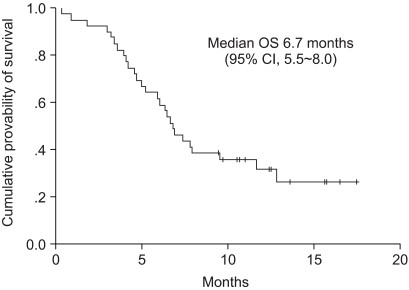
We conducted a multi-center, phase II trial to evaluate the efficacy and safety of using Padexol (a paclitaxel formulation) combined with cisplatin for the patients suffering with advanced gastric adenocarcinoma. MATERIALS AND METHODS: 39 patients (median age: 60 years; males: 90%) who were diagnosed with advanced gastric cancer were enrolled from 5 hospitals. Padexol 175 mg/m2 was administered as a 3-hr infusion, and this was followed by cisplatin 75 mg/m2 as an intravenous infusion on day 1, once every 3 weeks. RESULTS: Out of these 39 patients, 34 patients were assessable for treatment efficacy and 39 patients were assessable for the toxicity. Objective responses occurred in 13 patients (33%); 1 patient (3%) had a complete response and 12 patients (31%) had partial responses. 6 patients (15%) achieved a stable disease state. The median duration of response was 7.1 months, and the median time to progression and the overall survival were 4.8 months and 6.7 months, respectively. The major treatment-related adverse events were hematologic toxicity, including WHO grade 3 or 4 neutropenia in 13 patients (33%). However, febrile neutropenia occurred in only 1 patient and the non-hematologic toxicity was usually mild. CONCLUSION: The combination of Padexol and cisplatin was found to be active and it seems to be a relatively well-tolerated regimen for the treatment of advanced gastric cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Shin HR, Jung KW, Won YJ, Park JG. KCCR-affiliated Hospitals. 2002 Annual Report of the Korea Central Cancer Registry: based on registered data from 139 hospitals. Cancer Res Treat. 2004; 36:103–114.
Article2. Korean National Statistical Office. http://www.nso.go.kr.3. Glimelius B, Ekstrom K, Hoffman K, Graf W, Sjoden PO, Haglund U, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997; 8:163–168. PMID: 9093725.
Article4. Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993; 72:37–41. PMID: 8508427.
Article5. Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer. 1995; 71:587–591. PMID: 7533517.
Article6. Rowinsky EK, Cazenave LA, Donehower RC. Taxol: a novel investigational antimicrotubule agent. J Natl Cancer Inst. 1990; 82:1247–1259. PMID: 1973737.
Article7. McGuire WP, Rowinsky EK, Rosenshein NB, Grumbine FC, Ettinger DS, Armstrong DK, et al. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med. 1989; 111:273–279. PMID: 2569287.
Article8. Murphy WK, Fossella FV, Winn RJ, Shin DM, Hynes HE, Gross HM, et al. Phase II study of taxol in patients with untreated advanced non-small-cell lung cancer. J Natl Cancer Inst. 1993; 85:384–388. PMID: 8094466.
Article9. Holmes FA, Walters RS, Theriault RL, Forman AD, Newton LK, Raber MN, et al. Phase II trial of taxol, an active drug in the treatment of metastatic breast cancer. J Natl Cancer Inst. 1991; 83:1797–1805. PMID: 1683908.
Article10. Ajani JA, Fairweather J, Dumas P, Patt YZ, Pazdur R, Mansfield PF. Phase II study of taxol in patients with advanced gastric carcinoma. Cancer J Sci Am. 1998; 4:269–274. PMID: 9689986.11. Ohtsu A, Boku N, Tamura F, Muro K, Shimada Y, Saigenji K, et al. An early phase II study of a 3-hour infusion of paclitaxel for advanced gastric cancer. Am J Clin Oncol. 1998; 21:416–419. PMID: 9708646.
Article12. Kollmannsberger C, Quietzsch D, Haag C, Lingenfelser T, Schroeder M, Hartmann JT, et al. A phase II study of paclitaxel, weekly, 24-hour continous infusion 5-fluorouracil, folinic acid and cisplatin in patients with advanced gastric cancer. Br J Cancer. 2000; 83:458–462. PMID: 10945491.13. Yamada Y, Shirao K, Ohtsu A, Boku N, Hyodo I, Saitoh H, et al. Phase II trial of paclitaxel by three-hour infusion for advanced gastric cancer with short premedication for prophylaxis against paclitaxel-associated hypersensitivity reactions. Ann Oncol. 2001; 12:1133–1137. PMID: 11583196.
Article14. Kruijtzer CM, Boot H, Beijnen JH, Lochs HL, Parnis FX, Planting AS, et al. Weekly oral paclitaxel as first-line treatment in patients with advanced gastric cancer. Ann Oncol. 2003; 14:197–204. PMID: 12562644.
Article15. Gadgeel SM, Shields AF, Heilbrun LK, Labadidi S, Zalupski M, Chaplen R, et al. Phase II study of paclitaxel and carboplatin in patients with advanced gastric cancer. Am J Clin Oncol. 2003; 26:37–41. PMID: 12576922.
Article16. Park SR, Oh DY, Kim DW, Kim TY, Heo DS, Bang YJ, et al. A multi-center, late phase II clinical trial of Genexol (paclitaxel) and cisplatin for patients with advanced gastric cancer. Oncol Rep. 2004; 12:1059–1064. PMID: 15492793.
Article17. Chang MN, Therneau TM, Wieand HS, Cha SS. Designs for group sequential phase II clinical trials. Biometrics. 1987; 43:865–874. PMID: 3427171.
Article18. Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, et al. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol. 2000; 18:2648–2657. PMID: 10894863.
Article19. Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997; 15:261–267. PMID: 8996151.
Article20. Einzig AI, Neuberg D, Remick SC, Karp DD, O'Dwyer PJ, Stewart JA, et al. Phase II trial of docetaxel (Taxotere) in patients with adenocarcinoma of the upper gastrointestinal tract previously untreated with cytotoxic chemotherapy: the Eastern Cooperative Oncology Group (ECOG) results of protocol E1293. Med Oncol. 1996; 13:87–93. PMID: 9013471.
Article21. Ajani JA, Baker J, Pisters PW, Ho L, Mansfield PF, Feig BW, et al. CPT-11 plus cisplatin in patients with advanced, untreated gastric or gastroesophageal junction carcinoma: results of a phase II study. Cancer. 2002; 94:641–646. PMID: 11857295.22. Louvet C, Andre T, Tigaud JM, Gamelin E, Douillard JY, Brunet R, et al. Phase II study of oxaliplatin, fluorouracil, and folinic acid in locally advanced or metastatic gastric cancer patients. J Clin Oncol. 2002; 20:4543–4548. PMID: 12454110.
Article23. Chollet P, Schoffski P, Weigang-Kohler K, Schellens JH, Cure H, Pavlidis N, et al. Phase II trial with S-1 in chemotherapy-naive patients with gastric cancer. A trial performed by the EORTC Early Clinical Studies Group (ECSG). Eur J Cancer. 2003; 39:1264–1270. PMID: 12763215.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Phase II Study of Paclitaxel and Cisplatin as Salvage Therapy for Patients with Advanced or Metastatic Gastric Cancer
- A Phase II Trial of Paclitaxel, 5-fluorouracil (5-FU) and Cisplatin in Patients with Metastatic or Recurrent Gastric Cancer
- Phase II Study of Cisplatin, Ifosfamide . Paclitaxel (CIP) as Neoadjuvant Chemotherapy in Patients with Locally Advanced Cervical Carcinoma
- Combination chemotherapy with docetaxel and cisplatin as first-line treatment in advanced gastric cancer: is it a new effective chemotherapy?
- A Multi-Center, Phase II Clinical Trial of Genexol(R) (Paclitaxel) and Cisplatin for Patients with Non-Small Cell Lung Cancer