Cancer Res Treat.
2007 Jun;39(2):49-53.
Phase II Study of Docetaxel and Cisplatin as First-line Chemotherapy in Patients with Recurrent or Metastatic Gastric Cancer
- Affiliations
-
- 1Division of Hematology and Oncology, Department of Internal Medicine, Soonchunhyang University College of Medicine, Seoul, Korea. parkhs@hosp.sch. ac.kr
Abstract
-
PURPOSE: Palliative chemotherapy for patients with recurrent or metastatic gastric cancer has been shown to have a survival benefit. Docetaxel monotherapy has achieved appreciable results for treating gastric cancer. We investigated the clinical efficacy and feasibility of a docetaxel and cisplatin combination regimen for patients suffering with recurrent or metastatic gastric cancer.
MATERIALS AND METHODS
Patients with histologically proven, bidimensionally measurable lesions of recurrent or metastatic gastric cancer, and they had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2 and no prior palliative chemotherapy were eligible for this study. The combination chemotherapy regimen consisted of docetaxel 75 mg/m2 plus cisplatin 75 mg/m2 on day 1, and this was repeated every 3 weeks until disease progression.
RESULTS
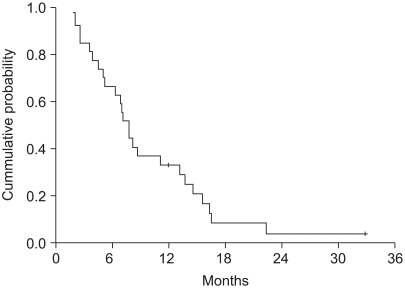
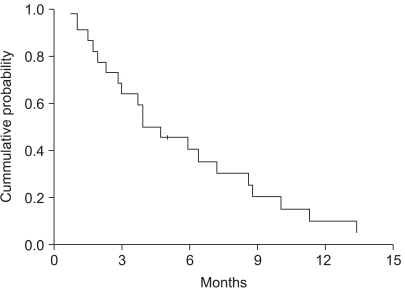
32 patients were enrolled from 2002 to 2005. The objective response rate was 31.3% (95% confidenceinterval (CI): 14.2~48.2%) with no CR. The disease control rate was 59.4%. At a median follow up of 38.9 months, the median overall survival was 7.4 months (95% CI: 6.3~8.5). The median time to progression was 4.7 months (95% CI: 3.1~6.3). During a total of 106 cycles, grade 3 or 4 hematological toxicities were observed as follows: neutropenia (39 of 106 cycles) and anemia (3 of 106 cycles). The grade 3 or 4 non-hematological toxicities included anorexia (18.9%) and nausea/vomiting (21.7%).
CONCLUSION
Docetaxel and cisplatin combination chemotherapy showed promising anti-tumor activity and this was well tolerated as a first-line treatment for patients with recurrent or metastatic gastric cancer. Further large, randomized phase III studies are warranted.
Keyword
MeSH Terms
Figure
Reference
-
1. Shin HR, Jung KW, Won YJ, Park JG. 139 KCCR-affiliated Hospitals. 2002 Annual Report of the Korea Central Cancer Registry: based on Registered Data from 139 Hospitals. Cancer Res Treat. 2004; 36:103–114.
Article2. Lacave AJ, Anton-Apariclo L, Gonzalez-Baron M. Cisplatin (CDDP) and 5-fluorouracil (5FU) 120-h infusion for advanced gastric cancer (GC): a phase II multicenter study. Proc Am Soc Clin Oncol. 1987; 6:91. (abstr).3. Preusser P, Wilke H, Achterrath W, Fink U, Lenaz L, Heinicke A, et al. Phase II study with the combination etoposide, doxorubicin, and cisplatin in advanced measurable gastric cancer. J Clin Oncol. 1989; 7:1310–1317. PMID: 2671287.
Article4. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981; 47:207–214. PMID: 7459811.
Article5. Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer. 1995; 71:587–591. PMID: 7533517.
Article6. Glimelius B, Ekstrom K, Hoffman K, Graf W, Sjoden PO, Haglund U, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997; 8:163–168. PMID: 9093725.
Article7. Roth AD, Maibach R, Martinelli G, Fazio N, Aapro MS, Pagani O, et al. Docetaxel (Taxotere)-cisplatin (TC): an effective drug combination in gastric carcinoma. Ann Oncol. 2000; 11:301–306. PMID: 10811496.
Article8. Ridwelski K, Gebauer T, Fahlke J, Kroning H, Kettener E, Meyer F, et al. Combination chemotherapy with docetaxel and cisplatin for locally advanced and metastatic gastric cancer. Ann Oncol. 2001; 12:47–51. PMID: 11249048.
Article9. Ajani JA, Fodor MB, Tjulandin SA, Moiseyenko VM, Chao Y, Cabral Filho S, et al. Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastroesophageal adenocarcinoma. J Clin Oncol. 2005; 23:5660–5667. PMID: 16110025.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combination chemotherapy with docetaxel and cisplatin as first-line treatment in advanced gastric cancer: is it a new effective chemotherapy?
- Curative Resection of Inoperable, Locally Advanced Gastric Cancer after Neoadjuvant Chemotherapy with Taxotere and Cisplatin
- Chemotherapy of Advanced Gastric Cancer
- Docetaxel plus cisplatin combination chemotherapy in patients with advanced gastric cancer
- Docetaxel and cisplatin combination chemotherapy for advanced gastric cancer failed to 5-fluorouracil-based chemotherapy