Cancer Res Treat.
2010 Dec;42(4):235-238.
A Case of Combined Hepatocellular-Cholangiocarcinoma with Favorable Response to Systemic Chemotherapy
- Affiliations
-
- 1Division of Oncology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. SSJ338@yuhs.ac
- 2Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
Abstract
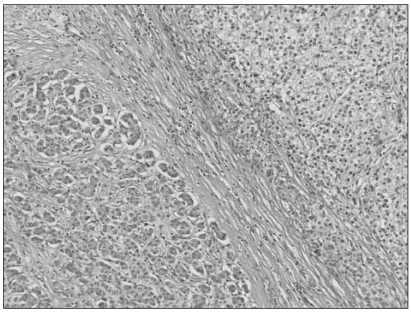
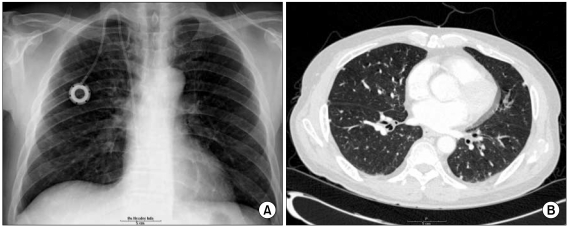
- Combined hepatocellular-cholangiocarcinoma (cHCC-CC) is a rare form of primary liver cancer composed of cells with histopathologic features of both hepatocellular carcinoma (HCC) and cholangiocarcinoma (CC). Because of its low incidence, the information on clinical outcomes of cHCC-CC is very limited and there are no published reports describing non-surgical treatment options for cHCC-CC. We report a case of cHCC-CC exhibiting a favorable response to systemic chemotherapy with doxorubicin and cisplatin. A 62-year-old man who recurred after a right lobectomy for cHCC-CC received sorafenib for palliative systemic therapy, but follow up imaging studies showed disease progression. He received 2nd line chemotherapy with doxorubicin at 60 mg/m2 together with cisplatin at 70 mg/m2. After 2 cycles of chemotherapy, a computed tomography scan of the chest showed markedly decreased size and number of the multiple lung metastases. After completing 8 cycles of 2nd line therapy, we changed the regimen to a fluorouracil (5-FU) mono therapy because of the toxicities associated with doxorubicin and cisplatin. To date, the patient has completed his 15th cycle of 5-FU mono therapy with the disease status remaining stable during 18 months of follow-up.
MeSH Terms
Figure
Reference
-
1. Goodman ZD, Ishak KG, Langloss JM, Sesterhenn IA, Rabin L. Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer. 1985; 55:124–135. PMID: 2578078.
Article2. Allen RA, Lisa JR. Combined liver cell and bile duct carcinoma. Am J Pathol. 1949; 25:647–655. PMID: 18152860.3. Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, et al. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer. 2002; 94:2040–2046. PMID: 11932907.4. Koh KC, Lee H, Choi MS, Lee JH, Paik SW, Yoo BC, et al. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg. 2005; 189:120–125. PMID: 15701504.
Article5. Hayashi H, Beppu T, Ishiko T, Mizumoto T, Masuda T, Okabe K, et al. A 42-month disease free survival case of combined hepatocellular-cholangiocarcinoma with lymph node metastases treated with multimodal therapy. Gan To Kagaku Ryoho. 2006; 33:1941–1943. PMID: 17212153.6. Kassahun WT, Hauss J. Management of combined hepatocellular and cholangiocarcinoma. Int J Clin Pract. 2008; 62:1271–1278. PMID: 18284443.
Article7. Tanaka S, Yamamoto T, Tanaka H, Kodai S, Ogawa M, Ichikawa T, et al. Potentiality of combined hepatocellular and intrahepatic cholangiocellular carcinoma originating from a hepatic precursor cell: Immunohistochemical evidence. Hepatol Res. 2005; 32:52–57. PMID: 15888382.
Article8. Yano Y, Yamamoto J, Kosuge T, Sakamoto Y, Yamasaki S, Shimada K, et al. Combined hepatocellular and cholangiocarcinoma: a clinicopathologic study of 26 resected cases. Jpn J Clin Oncol. 2003; 33:283–287. PMID: 12913082.
Article9. Taguchi J, Nakashima O, Tanaka M, Hisaka T, Takazawa T, Kojiro M. A clinicopathological study on combined hepatocellular and cholangiocarcinoma. J Gastroenterol Hepatol. 1996; 11:758–764. PMID: 8872774.
Article10. Maeda Y, Nishida M, Mori N, Tamesa T, Okada T, Tangoku A, et al. Combined hepatocellular carcinoma and cholangiocellular carcinoma. Nippon Rinsho. 2001; 59(Suppl 6):401–405. PMID: 11761981.11. Shimoda M, Kubota K. Multi-disciplinary treatment for cholangiocellular carcinoma. World J Gastroenterol. 2007; 13:1500–1504. PMID: 17461440.
Article12. Kim KH, Lee SG, Park EH, Hwang S, Ahn CS, Moon DB, et al. Surgical treatments and prognoses of patients with combined hepatocellular carcinoma and cholangiocarcinoma. Ann Surg Oncol. 2009; 16:623–629. PMID: 19130133.
Article13. Chan AC, Lo CM, Ng IO, Fan ST. Liver transplantation for combined hepatocellular cholangiocarcinoma. Asian J Surg. 2007; 30:143–146. PMID: 17475587.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combined Hepatocellular-Cholangiocarcinoma: Recent Progress in Pathology and Classification
- Treatment of advanced stage cholangiocarcinoma: Systemic therapy may be the starting step for radical surgery
- Combined Hepatocellular-cholangiocarcinoma
- Combined Hepatocellular-cholangiocarcinoma
- A Case of Curative Resection of Advanced Combined Hepatocellular-cholangiocarcinoma after Neoadjuvant Chemotherapy