J Pathol Transl Med.
2016 May;50(3):225-230. 10.4132/jptm.2016.03.08.
Investigation of the Roles of Cyclooxygenase-2 and Galectin-3 Expression in the Pathogenesis of Premenopausal Endometrial Polyps
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Sifa University School of Medicine, Izmir, Turkey. dresincelik@windowslive.com
- 2Department of Patology, Sifa University School of Medicine, Izmir, Turkey.
- KMID: 2164600
- DOI: http://doi.org/10.4132/jptm.2016.03.08
Abstract
- BACKGROUND
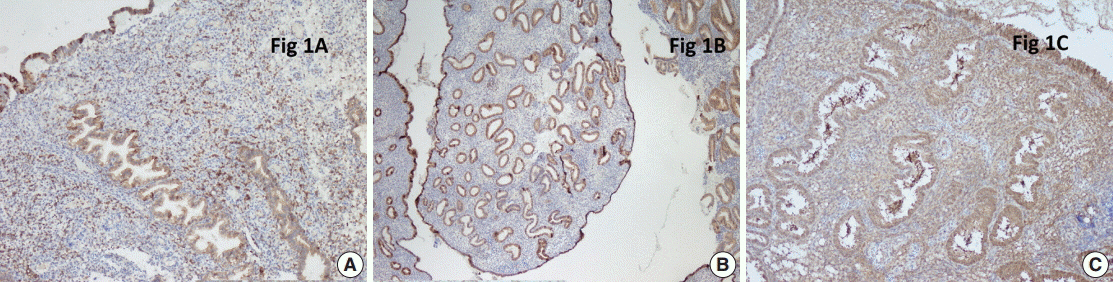
The pathogenesis and etiology of endometrial polyps has not been elucidated. In this study, we aimed to examine the pathogenic mechanisms of endometrial polyp development using immunohistochemistry. We evaluated the expression of galectin-3 and cyclooxgenase-2 (COX-2) during the menstrual cycle in premenopausal women with endometrial polyps or normal endometrium.
METHODS
Thirty-one patients with endometrial polyps and 50 healthy control patients were included in this study. The levels of expression of COX-2 and galectin-3 were studied by immunohistochemistry.
RESULTS
The percentage of COX-2-positive cells and the intensity of COX-2 staining in the endometrium did not vary during the menstrual cycle either in the control group or in patients with endometrial polyps. However, expression of galectin-3 was significantly lower in endometrial polyps and during the proliferative phase of the endometrium compared with the secretory phase.
CONCLUSIONS
Our data suggests that the pathogenesis of endometrial polyps does not involve expression of COX-2 or galectin-3.
MeSH Terms
Figure
Reference
-
1. Neto LC, Soares JM Jr, Giusa-Chiferi MG, Gonçalves WJ, Baracat EC. Expression of p53 protein in the endometrial polyp in postmenopausal women. Eur J Gynaecol Oncol. 2013; 34:509–12.2. Clevenger-Hoeft M, Syrop CH, Stovall DW, Van Voorhis BJ. Sonohysterography in premenopausal women with and without abnormal bleeding. Obstet Gynecol. 1999; 94:516–20.
Article3. Droegemueller W. Benign gynecologic lesions. In : Stenchever MA, Droegemueller W, Herbst AL, Mishell DR, editors. Comprehensive gynecology. 5th ed. Louis: Mosby Inc;2001. p. 440–92.4. Inceboz US, Nese N, Uyar Y, et al. Hormone receptor expressions and proliferation markers in postmenopausal endometrial polyps. Gynecol Obstet Invest. 2006; 61:24–8.
Article5. Sant’Ana de Almeida EC, Nogueira AA, Candido dos Reis FJ, Zambelli Ramalho LN, Zucoloto S. Immunohistochemical expression of estrogen and progesterone receptors in endometrial polyps and adjacent endometrium in postmenopausal women. Maturitas. 2004; 49:229–33.
Article6. Kendall RL, Wang G, Thomas KA. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem Biophys Res Commun. 1996; 226:324–8.
Article7. Lee J, Moon C, Kim J, et al. Immunohistochemical localization of galectin-3 in the granulomatous lesions of paratuberculosis-infected bovine intestine. J Vet Sci. 2009; 10:177–80.
Article8. Sakaki M, Fukumori T, Fukawa T, et al. Clinical significance of galectin-3 in clear cell renal cell carcinoma. J Med Invest. 2010; 57:152–7.
Article9. Topcu HO, Erkaya S, Guzel AI, et al. Risk factors for endometrial hyperplasia concomitant endometrial polyps in pre- and postmenopausal women. Asian Pac J Cancer Prev. 2014; 15:5423–5.
Article10. Costa-Paiva L, Godoy CE Jr, Antunes A Jr, Caseiro JD, Arthuso M, Pinto-Neto AM. Risk of malignancy in endometrial polyps in premenopausal and postmenopausal women according to clinicopathologic characteristics. Menopause. 2011; 18:1278–82.
Article11. Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950; 1:3–25.
Article12. Harrington DJ, Lessey BA, Rai V, et al. Tenascin is differentially expressed in endometrium and endometriosis. J Pathol. 1999; 187:242–8.
Article13. Maia H Jr, Maltez A, Studard E, Zausner B, Athayde C, Coutinho E. Effect of the menstrual cycle and oral contraceptives on cyclooxygenase-2 expression in the endometrium. Gynecol Endocrinol. 2005; 21:57–61.14. Erdemoglu E, Güney M, Karahan N, Mungan T. Expression of cyclooxygenase-2, matrix metalloproteinase-2 and matrix metalloproteinase-9 in premenopausal and postmenopausal endometrial polyps. Maturitas. 2008; 59:268–74.
Article15. Bulun SE, Zeitoun KM, Takayama K, Sasano H. Estrogen biosynthesis in endometriosis: molecular basis and clinical relevance. J Mol Endocrinol. 2000; 25:35–42.
Article16. Wang D, Chen Q, Zhang C, Ren F, Li T. DNA hypomethylation of the COX-2 gene promoter is associated with up-regulation of its mRNA expression in eutopic endometrium of endometriosis. Eur J Med Res. 2012; 17:12.
Article17. Carvalho FM, Aguiar FN, Tomioka R, de Oliveira RM, Frantz N, Ueno J. Functional endometrial polyps in infertile asymptomatic patients: a possible evolution of vascular changes secondary to endometritis. Eur J Obstet Gynecol Reprod Biol. 2013; 170:152–6.
Article18. Maia H Jr, Correia T, Freitas LA, Athayde C, Coutinho E. Cyclooxygenase-2 expression in endometrial polyps during menopause. Gynecol Endocrinol. 2005; 21:336–9.
Article19. Maia H Jr, Pimentel K, Silva TM, et al. Aromatase and cyclooxygenase-2 expression in endometrial polyps during the menstrual cycle. Gynecol Endocrinol. 2006; 22:219–24.
Article20. Pinheiro A, Antunes A Jr, Andrade L, De Brot L, Pinto-Neto AM, Costa-Paiva L. Expression of hormone receptors, Bcl2, Cox2 and Ki67 in benign endometrial polyps and their association with obesity. Mol Med Rep. 2014; 9:2335–41.
Article21. Du GP, Zhang W, Wang L, Liu YK, Zhou JP. Expression of galectin-3 in human endometrium. Fudan Univ J Med Sci. 2006; 2:143–6.22. Chiu CG, Strugnell SS, Griffith OL, et al. Diagnostic utility of galectin-3 in thyroid cancer. Am J Pathol. 2010; 176:2067–81.
Article23. Cay T. Immunhistochemical expression of galectin-3 in cancer: a review of the literature. Turk Patoloji Derg. 2012; 28:1–10.
Article24. Nöel JC, Chapron C, Borghese B, Fayt I, Anaf V. Galectin-3 is overexpressed in various forms of endometriosis. Appl Immunohistochem Mol Morphol. 2011; 19:253–7.25. Brustmann H, Riss D, Naudé S. Galectin-3 expression in normal, hyperplastic, and neoplastic endometrial tissues. Pathol Res Pract. 2003; 199:151–8.
Article26. Silverberg SG, Kurman RJ. Atlas of tumor pathology, 3rd series. Fascicle 3. Tumors of the uterine corpus and gestational trophoblastic disease. Washington, DC: Armed Forces Institute of Pathology;1992.27. Yang H, Lei C, Cheng C, et al. The antiapoptotic effect of galectin-3 in human endometrial cells under the regulation of estrogen and progesterone. Biol Reprod. 2012; 87:39.
Article28. Kumar V, Abbas AK, Fausto N. Robbins and Cotran: pathologic basis of disease. 7th ed. Philadelphia: Elsevier Saunders;2005. p. 269–342.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Risk Factors Associated with Endometrial Pathology in Premenopausal Breast Cancer Patients Treated with Tamoxifen
- Detection of changes in endometrial polyps by sonohysterography
- Endometrial polyps: Is the prediction of spontaneous regression possible?
- Endometrial polyp surveillance in premenopausal breast cancer patients using tamoxifen
- Comparison of cyclooxygenase-2 and p53 expression in normal endometrium, endometrial hyperplasia and endometrial cancer