J Vet Sci.
2015 Jun;16(2):157-164. 10.4142/jvs.2015.16.2.157.
Preparation and evaluation of enrofloxacin microspheres and tissue distribution in rats
- Affiliations
-
- 1Laboratory of Veterinary Pharmacology, College of Animal Science and Technology, Henan University of Science and Technology, Luoyang 471003, China.
- 2Laboratory of Veterinary Pharmacology, College of Veterinary Medicine, South China Agricultural University, Guangzhou 510642, China. zlzeng@scau.edu.cn
- 3Ceva Animal Health Service Company Limited, Beijing 100016, China.
- KMID: 2164524
- DOI: http://doi.org/10.4142/jvs.2015.16.2.157
Abstract
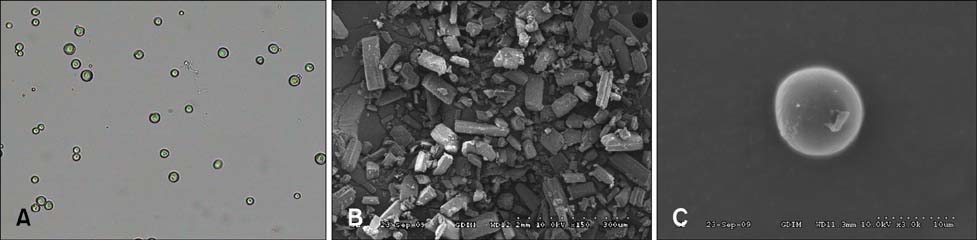
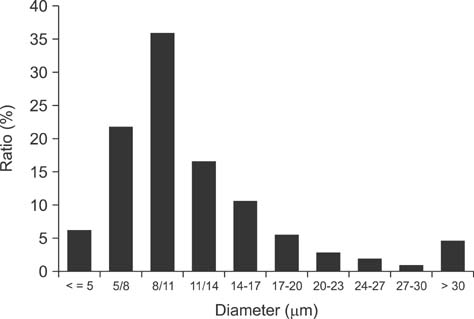
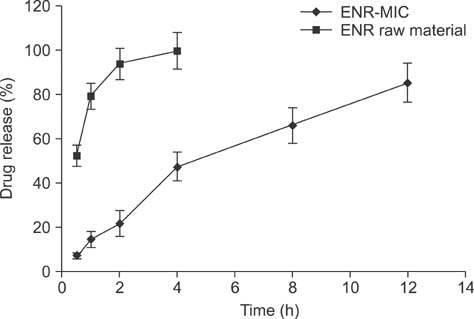
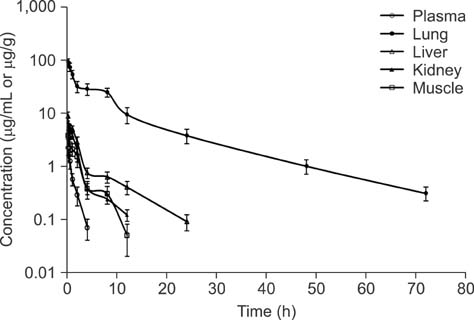
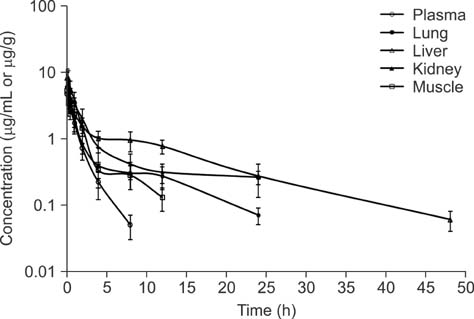
- New enrofloxacin microspheres were formulated, and their physical properties, lung-targeting ability, and tissue distribution in rats were examined. The microspheres had a regular and round shape. The mean diameter was 10.06 microm, and the diameter of 89.93% of all microspheres ranged from 7.0 microm to 30.0 microm. Tissue distribution of the microspheres was evaluated along with a conventional enrofloxacin preparation after a single intravenous injection (7.5 mg of enrofloxacin/kg bw). The results showed that the elimination half-life (t(1/2beta)) of enrofloxacin from lung was prolonged from 7.94 h for the conventional enrofloxacin to 13.28 h for the microspheres. Area under the lung concentration versus time curve from 0 h to infinity (AUC(0-infinity)) was increased from 11.66 h.microg/g to 508.00 h.microg/g. The peak concentration (Cmax) in lung was increased from 5.95 microg/g to 93.36 microg/g. Three lung-targeting parameters were further assessed and showed that the microspheres had remarkable lung-targeting capabilities.
MeSH Terms
Figure
Reference
-
1. Bebear CM, Renaudin J, Charron A, Renaudin H, de Barbeyrac B, Schaeverbeke T, Bebear C. Mutations in the gyrA, parC, and parE genes associated with fluoroquinolone resistance in clinical isolates of Mycoplasma hominis. Antimicrob Agents Chemother. 1999; 43:954–956.
Article2. Bimazubute M, Cambier C, Baert K, Vanbelle S, Chiap P, Albert A, Delporte JP, Gustin P. Penetration of enrofloxacin into the nasal secretions and relationship between nasal secretions and plasma enrofloxacin concentrations after intramuscular administration in healthy pigs. J Vet Pharmacol Ther. 2010; 33:183–188.
Article3. Bregante MA, Saez P, Aramayona JJ, Fraile L, Garcia MA, Solans C. Comparative pharmacokinetics of enrofloxacin in mice, rats, rabbits, sheep, and cows. Am J Vet Res. 1999; 60:1111–1116.4. Brown SA. Fluoroquinolones in animal health. J Vet Pharmacol Ther. 1996; 19:1–14.
Article5. Cambier C, Bimazubute M, Baert K, Vanbelle S, Chiap P, Albert A, Delporte JP, Gustin P. Penetration of enrofloxacin into the nasal secretions in healthy pigs. Relationship between nasal secretions and plasma enrofloxacin concentrations. J Vet Pharmacol Ther. 2009; 32:Suppl 1. 147–148.6. Dorfman M, Barsanti J, Budsberg SC. Enrofloxacin concentrations in dogs with normal prostate and dogs with chronic bacterial prostatitis. Am J Vet Res. 1995; 56:386–390.7. El-Sherbiny IM, Smyth HDC. Biodegradable nano-micro carrier systems for sustained pulmonary drug delivery: (I) self-assembled nanoparticles encapsulated in respirable/swellable semi-IPN microspheres. Int J Pharm. 2010; 395:132–141.
Article8. Gautier-Bouchardon AV, Reinhardt AK, Kobisch M, Kempf I. In vitro development of resistance to enrofloxacin, erythromycin, tylosin, tiamulin and oxytetracycline in Mycoplasma gallisepticum, Mycoplasma iowae and Mycoplasma synoviae. Vet Microbiol. 2002; 88:47–58.
Article9. Genchi M, Pengo G, Genchi C. Efficacy of moxidectin microsphere sustained release formulation for the prevention of subcutaneous filarial (Dirofilaria repens) infection in dogs. Vet Parasitol. 2010; 170:167–169.
Article10. Gupta PK, Hung CT. Quantitative evaluation of targeted drug delivery systems. Int J Pharm. 1989; 56:217–226.
Article11. Haritova A, Urumova V, Lutckanov M, Petrov V, Lashev L. Pharmacokinetic-pharmacodynamic indices of enrofloxacin in Escherichia coli O78/H12 infected chickens. Food Chem Toxicol. 2011; 49:1530–1536.
Article12. Ilium L, Davis SS, Wilson CG, Thomas NW, Frier M, Hardy JG. Blood clearance and organ deposition of intravenously administered colloidal particles. The effects of particle size, nature and shape. Int J Pharm. 1982; 12:135–146.
Article13. Kalpana S, Aggarwal M, Srinivasa Rao G, Malik JK. Effects of aflatoxin B1 on tissue residues of enrofloxacin and its metabolite ciprofloxacin in broiler chickens. Environ Toxicol Pharmacol. 2012; 33:121–126.
Article14. Keil DJ, Fenwick B. Role of Bordetella bronchiseptica in infectious tracheobronchitis in dogs. J Am Vet Med Assoc. 1998; 212:200–207.15. Lagarce F, Faisant N, Desfontis JC, Marescaux L, Gautier F, Richard J, Menei P, Benoit JP. Baclofen-loaded microspheres in gel suspensions for intrathecal drug delivery: in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2005; 61:171–180.
Article16. Lam XM, Duenas ET, Daugherty AL, Levin N, Cleland JL. Sustained release of recombinant human insulin-like growth factor-I for treatment of diabetes. J Control Release. 2000; 67:281–292.
Article17. Lizondo M, Pons M, Gallardo M, Estelrich J. Physicochemical properties of enrofloxacin. J Pharm Biomed Anal. 1997; 15:1845–1849.
Article18. Mu L, Feng SS. A novel controlled release formulation for the anticancer drug paclitaxel (Taxol®): PLGA nanoparticles containing vitamin E TPGS. J Control Release. 2003; 86:33–48.
Article19. Nouaille-Degorce B, Veau C, Dautrey S, Tod M, Laouari D, Carbon C, Farinotti R. Influence of renal failure on ciprofloxacin pharmacokinetics in rats. Antimicrob Agents Chemother. 1998; 42:289–292.
Article20. Rahal A, Kumar A, Ahmad AH, Malik JK, Ahuja V. Pharmacokinetics of enrofloxacin in sheep following intravenous and subcutaneous administration. J Vet Pharmacol Ther. 2006; 29:321–324.
Article21. Sahin S, Selek H, Ponchel G, Ercan MT, Sargon M, Hincal AA, Kas HS. Preparation, characterization and in vivo distribution of terbutaline sulfate loaded albumin microspheres. J Control Release. 2002; 82:345–358.
Article22. Schettini DA, Ribeiro RR, Demicheli C, Rocha OGF, Melo MN, Michalick MSM, Frézard F. Improved targeting of antimony to the bone marrow of dogs using liposomes of reduced size. Int J Pharm. 2006; 315:140–147.
Article23. Schmidt S, Müller RH. Plasma protein adsorption patterns on surfaces of Amphotericin B-containing fat emulsions. Int J Pharm. 2003; 254:3–5.
Article24. Schroder J. Enrofloxacin: a new antimicrobial agent. J S Afr Vet Assoc. 1989; 60:122–124.25. Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997; 28:5–24.
Article26. Son JS, Appleford M, Ong JL, Wenke JC, Kim JM, Choi SH, Oh DS. Porous hydroxyapatite scaffold with three-dimensional localized drug delivery system using biodegradable microspheres. J Control Release. 2011; 153:133–140.
Article27. Tang S, Zhou Y, Li R, Chen Q, Xiao X. Pharmacokinetics and lung-targeting characterization of a newly formulated enrofloxacin preparation. J Vet Pharmacol Ther. 2007; 30:443–450.
Article28. Wu CC, Shryock TR, Lin TL, Faderan M, Veenhuizen MF. Antimicrobial susceptibility of Mycoplasma hyorhinis. Vet Microbiol. 2000; 76:25–30.29. Ye J, Wang Q, Zhou X, Zhang N. Injectable actarit-loaded solid lipid nanoparticles as passive targeting therapeutic agents for rheumatoid arthritis. Int J Pharm. 2008; 352:273–279.
Article30. Yi H, Shin MJ, Cho SM, Lee DG, Cho K, Cho HJ, Shin SJ, Bartlett MG, Kim JS, Shin HC. Nonlinear toxicokinetics of enrofloxacin in rats. Arch Pharm Res. 2010; 33:1851–1857.
Article31. Zeng ZL, Huang XH, Ding HZ, Zhen ZL. A comparison of pharmacokinetics in pigs after intramuscular administration of two different pH enrofloxacin injections. Chinese J Vet Drug. 2001; 35:13–15.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pharmacoknetic Study of Diclofenac and Its Interaction with Enrofloxacin in Buffalo Calves
- Effects of Bone Density as Injections of Salmon Calcitonin Micropheres in Ovariectomized Rats
- Modulatory action of enrofloxacin in lipopolysaccharide-induced hyper-activated mouse spleen cells
- Prolonged Regional Anesthesia with Lidocaine Microspheres by Using a Biodegradable Polymer
- Porous Microcarrier-Enabled Three-Dimensional Culture of Chondrocytes for Cartilage Engineering: A Feasibility Study