J Korean Med Sci.
2015 Aug;30(8):1157-1166. 10.3346/jkms.2015.30.8.1157.
Epigenetic Role of Histone 3 Lysine Methyltransferase and Demethylase in Regulating Apoptosis Predicting the Recurrence of Atypical Meningioma
- Affiliations
-
- 1Department of Neurosurgery and Division of Neurooncology, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea. yzkim@skku.edu
- 2Department of Pathology, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- 3Department of Molecular and Cellular Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
- 4Department of Neurosurgery, Dong-A University Medical Center, Dong-A University College of Medicine, Busan, Korea.
- KMID: 2164512
- DOI: http://doi.org/10.3346/jkms.2015.30.8.1157
Abstract
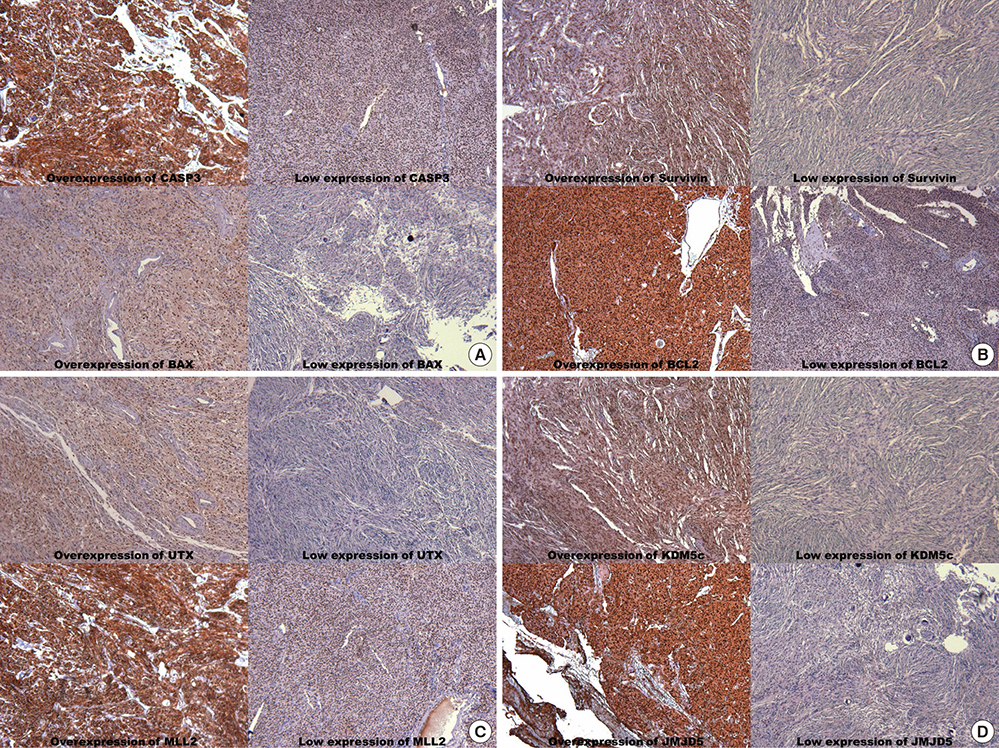
- Alteration of apoptosis is related with progression and recurrence of atypical meningiomas (AMs). However, no comprehensive study has been conducted regarding histone modification regulating apoptosis in AMs. This study aimed to determine the prognostic values of certain apoptosis-associated factors, and examine the role of histone modification on apoptosis in AMs. The medical records of 67 patients with AMs, as diagnosed during recent 13 yr, were reviewed retrospectively. Immunohistochemical staining was performed on archived paraffin-embedded tissues for pro-apoptotic factors (CASP3, IGFBP, TRAIL-R1, BAX, and XAF1), anti-apoptotic factors (survivin, ERK, RAF1, MDM2, and BCL2), and the histone modifying enzymes (MLL2, RIZ, EZH1, NSD2, KDM5c, JMJD2a, UTX, and JMJD5). Twenty-six (38.8%) patients recurred during the follow-up period (mean duration 47.7 months). In terms of time-to-recurrence (TTR), overexpression of CASP3, TRAIL-R1, and BAX had a longer TTR than low expression, and overexpression of survivin, MDM2, and BCL2 had a shorter TTR than low expression (P<0.05). Additionally, overexpression of MLL2, UTX, and JMJ5 had shorter TTRs than low expression, and overexpression of KDM5c had a longer TTR than low expression. However, in the multi-variate analysis of predicting factors for recurrence, low expression of CASP3 (P<0.001), and BAX (P<0.001), and overexpression of survivin (P=0.007), and MDM2 (P=0.037) were associated with recurrence independently, but any enzymes modifying histone were not associated with recurrence. Conclusively, this study suggests certain apoptosis-associated factors should be associated with recurrence of AMs, which may be regulated epigenetically by histone modifying enzymes.
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Apoptosis/*genetics
Apoptosis Regulatory Proteins/genetics
Epigenesis, Genetic/genetics
Female
Gene Expression Regulation, Neoplastic/genetics
Histone Code/genetics
Histone Demethylases/*genetics
Histone-Lysine N-Methyltransferase/*genetics
Humans
Longitudinal Studies
Male
Meningeal Neoplasms/*genetics/pathology
Meningioma/*genetics/pathology
Middle Aged
Neoplasm Recurrence, Local/*genetics
Apoptosis Regulatory Proteins
Histone Demethylases
Histone-Lysine N-Methyltransferase
Figure
Reference
-
1. Brat DJ, Parisi JE, Kleinschmidt-DeMasters BK, Yachnis AT, Montine TJ, Boyer PJ, Powell SZ, Prayson RA, McLendon RE. Neuropathology Committee, College of American Pathologists. Surgical neuropathology update: a review of changes introduced by the WHO classification of tumours of the central nervous system, 4th edition. Arch Pathol Lab Med. 2008; 132:993–1007.2. Pearson BE, Markert JM, Fisher WS, Guthrie BL, Fiveash JB, Palmer CA, Riley K. Hitting a moving target: evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg Focus. 2008; 24:E3.3. Rogers L, Gilbert M, Vogelbaum MA. Intracranial meningiomas of atypical (WHO grade II) histology. J Neurooncol. 2010; 99:393–405.4. Moon HS, Jung S, Jang WY, Jung TY, Moon KS, Kim IY. Intracranial meningiomas, WHO Grade II: prognostic implications of clinicopathologic features. J Korean Neurosurg Soc. 2012; 52:14–20.5. Marosi C, Hassler M, Roessler K, Reni M, Sant M, Mazza E, Vecht C. Meningioma. Crit Rev Oncol Hematol. 2008; 67:153–171.6. Tanzler E, Morris CG, Kirwan JM, Amdur RJ, Mendenhall WM. Outcomes of WHO Grade I meningiomas receiving definitive or postoperative radiotherapy. Int J Radiat Oncol Biol Phys. 2011; 79:508–513.7. Choy W, Kim W, Nagasawa D, Stramotas S, Yew A, Gopen Q, Parsa AT, Yang I. The molecular genetics and tumor pathogenesis of meningiomas and the future directions of meningioma treatments. Neurosurg Focus. 2011; 30:E6.8. Kane AJ, Sughrue ME, Rutkowski MJ, Shangari G, Fang S, McDermott MW, Berger MS, Parsa AT. Anatomic location is a risk factor for atypical and malignant meningiomas. Cancer. 2011; 117:1272–1278.9. Pasquier D, Bijmolt S, Veninga T, Rezvoy N, Villa S, Krengli M, Weber DC, Baumert BG, Canyilmaz E, Yalman D, et al. Rare Cancer Network. Atypical and malignant meningioma: outcome and prognostic factors in 119 irradiated patients. A multicenter, retrospective study of the Rare Cancer Network. Int J Radiat Oncol Biol Phys. 2008; 71:1388–1393.10. Yamasaki F, Yoshioka H, Hama S, Sugiyama K, Arita K, Kurisu K. Recurrence of meningiomas. Cancer. 2000; 89:1102–1110.11. Komotar RJ, Iorgulescu JB, Raper DM, Holland EC, Beal K, Bilsky MH, Brennan CW, Tabar V, Sherman JH, Yamada Y, et al. The role of radiotherapy following gross-total resection of atypical meningiomas. J Neurosurg. 2012; 117:679–686.12. Stessin AM, Schwartz A, Judanin G, Pannullo SC, Boockvar JA, Schwartz TH, Stieg PE, Wernicke AG. Does adjuvant external-beam radiotherapy improve outcomes for nonbenign meningiomas? a surveillance, epidemiology, and end results (SEER)-based analysis. J Neurosurg. 2012; 117:669–675.13. Alama A, Barbieri F, Spaziante R, Bruzzo C, Dadati P, Dorcaratto A, Ravetti JL. Significance of cyclin D1 expression in meningiomas: a preliminary study. J Clin Neurosci. 2007; 14:355–358.14. Konstantinidou AE, Pavlopoulos PM, Patsouris E, Kaklamanis L, Davaris P. Expression of apoptotic and proliferation markers in meningiomas. J Pathol. 1998; 186:325–330.15. Ho DM, Hsu CY, Ting LT, Chiang H. Histopathology and MIB-1 labeling index predicted recurrence of meningiomas: a proposal of diagnostic criteria for patients with atypical meningioma. Cancer. 2002; 94:1538–1547.16. Maes L, Lippens E, Kalala JP, de Ridder L. The hTERT-protein and Ki-67 labelling index in recurrent and non-recurrent meningiomas. Cell Prolif. 2005; 38:3–12.17. Hopkins-Donaldson S, Bodmer JL, Bourloud KB, Brognara CB, Tschopp J, Gross N. Loss of caspase-8 expression in highly malignant human neuroblastoma cells correlates with resistance to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. Cancer Res. 2000; 60:4315–4319.18. Lowe SW, Jacks T, Housman DE, Ruley HE. Abrogation of oncogene-associated apoptosis allows transformation of p53-deficient cells. Proc Natl Acad Sci U S A. 1994; 91:2026–2030.19. Kayaselçuk F, Zorludemir S, Bal N, Erdogan B, Erdogan S, Erman T. The expression of survivin and Ki-67 in meningiomas: correlation with grade and clinical outcome. J Neurooncol. 2004; 67:209–214.20. Konstantinidou AE, Givalos N, Gakiopoulou H, Korkolopoulou P, Kotsiakis X, Boviatsis E, Agrogiannis G, Mahera H, Patsouris E. Caspase-3 immunohistochemical expression is a marker of apoptosis, increased grade and early recurrence in intracranial meningiomas. Apoptosis. 2007; 12:695–705.21. Strasser A, O'Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000; 69:217–245.22. Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998; 281:1322–1326.23. Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999; 399:483–487.24. Tang D, Kidd VJ. Cleavage of DFF-45/ICAD by multiple caspases is essential for its function during apoptosis. J Biol Chem. 1998; 273:28549–28552.25. Perry A, Louis DN, Scheithauer BW, Budka H, von Deimling A. Meningiomas. In : Louis DN, Deutsches Krebsforschungszentrum H, editors. International Agency for Research on Cancer. World Health Organization. WHO classification of tumours of the central nervous system. New York: International Agency for Research on Cancer;2007. p. 164–172.26. Eng J. Receiver operating characteristic analysis: a primer. Acad Radiol. 2005; 12:909–916.27. Patel T, Gores GJ, Kaufmann SH. The role of proteases during apoptosis. FASEB J. 1996; 10:587–597.28. Vermeulen K, Van Bockstaele DR, Berneman ZN. Apoptosis: mechanisms and relevance in cancer. Ann Hematol. 2005; 84:627–639.29. Schwerk C, Schulze-Osthoff K. Non-apoptotic functions of caspases in cellular proliferation and differentiation. Biochem Pharmacol. 2003; 66:1453–1458.30. Sasaki T, Lopes MB, Hankins GR, Helm GA. Expression of survivin, an inhibitor of apoptosis protein, in tumors of the nervous system. Acta Neuropathol. 2002; 104:105–109.31. Scott FL, Denault JB, Riedl SJ, Shin H, Renatus M, Salvesen GS. XIAP inhibits caspase-3 and -7 using two binding sites: evolutionarily conserved mechanism of IAPs. EMBO J. 2005; 24:645–655.32. Matsumori Y, Northington FJ, Hong SM, Kayama T, Sheldon RA, Vexler ZS, Ferriero DM, Weinstein PR, Liu J. Reduction of caspase-8 and -9 cleavage is associated with increased c-FLIP and increased binding of Apaf-1 and Hsp70 after neonatal hypoxic/ischemic injury in mice overexpressing Hsp70. Stroke. 2006; 37:507–512.33. Sabbatini M, Comi C, Chiocchetti A, Piffanelli V, Car PG, Dianzani U, Monaco F, Cannas M. Signals of apoptotic pathways in several types of meningioma. Pathol Oncol Res. 2011; 17:51–59.34. Adida C, Berrebi D, Peuchmaur M, Reyes-Mugica M, Altieri DC. Anti-apoptosis gene, survivin, and prognosis of neuroblastoma. Lancet. 1998; 351:882–883.35. Ambrosini G, Adida C, Sirugo G, Altieri DC. Induction of apoptosis and inhibition of cell proliferation by survivin gene targeting. J Biol Chem. 1998; 273:11177–11182.36. Asanuma K, Moriai R, Yajima T, Yagihashi A, Yamada M, Kobayashi D, Watanabe N. Survivin as a radioresistance factor in pancreatic cancer. Jpn J Cancer Res. 2000; 91:1204–1209.37. Angileri FF, Aguennouz M, Conti A, La Torre D, Cardali S, Crupi R, Tomasello C, Germanò A, Vita G, Tomasello F. Nuclear factor-kappaB activation and differential expression of survivin and Bcl-2 in human grade 2-4 astrocytomas. Cancer. 2008; 112:2258–2266.38. Mellai M, Caldera V, Patrucco A, Annovazzi L, Schiffer D. Survivin expression in glioblastomas correlates with proliferation, but not with apoptosis. Anticancer Res. 2008; 28:109–118.39. Lee MG, Villa R, Trojer P, Norman J, Yan KP, Reinberg D, Di Croce L, Shiekhattar R. Demethylation of H3K27 regulates polycomb recruitment and H2A ubiquitination. Science. 2007; 318:447–450.40. Shen Y, Guo X, Wang Y, Qiu W, Chang Y, Zhang A, Duan X. Expression and significance of histone H3K27 demethylases in renal cell carcinoma. BMC Cancer. 2012; 12:470.41. Murati A, Brecqueville M, Devillier R, Mozziconacci MJ, Gelsi-Boyer V, Birnbaum D. Myeloid malignancies: mutations, models and management. BMC Cancer. 2012; 12:304.42. Jones DT, Jäger N, Kool M, Zichner T, Hutter B, Sultan M, Cho YJ, Pugh TJ, Hovestadt V, Stütz AM, et al. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012; 488:100–105.43. Yamamoto H, Watanabe Y, Maehata T, Morita R, Yoshida Y, Oikawa R, Ishigooka S, Ozawa S, Matsuo Y, Hosoya K, et al. An updated review of gastric cancer in the next-generation sequencing era: insights from bench to bedside and vice versa. World J Gastroenterol. 2014; 20:3927–3937.44. Paolicchi E, Crea F, Farrar WL, Green JE, Danesi R. Histone lysine demethylases in breast cancer. Crit Rev Oncol Hematol. 2013; 86:97–103.45. Kim JH, Sharma A, Dhar SS, Lee SH, Gu B, Chan CH, Lin HK, Lee MG. UTX and MLL4 coordinately regulate transcriptional programs for cell proliferation and invasiveness in breast cancer cells. Cancer Res. 2014; 74:1705–1717.46. Xia M, Xu L, Leng Y, Gao F, Xia H, Zhang D, Ding X. Downregulation of MLL3 in esophageal squamous cell carcinoma is required for the growth and metastasis of cancer cells. Tumour Biol. 2015; 36:605–613.47. Park HJ, Kang HC, Kim IH, Park SH, Kim DG, Park CK, Paek SH, Jung HW. The role of adjuvant radiotherapy in atypical meningioma. J Neurooncol. 2013; 115:241–247.48. Kim MS, Kim KH, Lee EH, Lee YM, Lee SH, Kim HD, Kim YZ. Results of immunohistochemical staining for cell cycle regulators predict the recurrence of atypical meningiomas. J Neurosurg. 2014; 121:1189–1200.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Links between Serine Biosynthesis Pathway and Epigenetics in Cancer Metabolism
- Epigenetic Cross-Talk between DNA Methylation and Histone Modifications in Human Cancers
- Genome-wide identification of histone lysine methyltransferases and their implications in the epigenetic regulation of eggshell formation-related genes in a trematode parasite Clonorchis sinensis
- The Histone Lysine-specific Demethylase 1 Inhibitor, SP2509 Exerts Cytotoxic Effects against Renal Cancer Cells through Downregulation of Bcl-2 and Mcl-1
- Inhibition of Nuclear Receptor Binding SET Domain 2/Multiple Myeloma SET Domain by LEM-06 Implication for Epigenetic Cancer Therapies