Int J Stem Cells.
2016 May;9(1):70-78. 10.15283/ijsc.2016.9.1.70.
Amniotic Fluid-Derived Mesenchymal Stem Cells Cut Short the Acuteness of Cisplatin-Induced Nephrotoxicity in Sprague-Dawley Rats
- Affiliations
-
- 1Department of Pathology, Faculty of Medicine, Mansoura University, Mansoura, Egypt.
- 2Zoology Unit-, Faculty of Medicine, Mansoura University, Mansoura, Egypt.
- 3Urology and Nephrology Center, Faculty of Medicine, Mansoura University, Mansoura, Egypt.
- 4Department of Clinical Pharmacology, Faculty of Medicine, Mansoura University, Mansoura, Egypt. zohoor26203@yahoo.com
- 5Medical Experimental Research Center (MERC), Faculty of Medicine, Mansoura University, Mansoura, Egypt.
- 6Department of Public Health, Faculty of Medicine, Mansoura University, Mansoura, Egypt.
- 7Department of Clinical Pathology, Faculty of Medicine, Mansoura University, Mansoura, Egypt.
- KMID: 2164163
- DOI: http://doi.org/10.15283/ijsc.2016.9.1.70
Abstract
- BACKGROUND AND OBJECTIVES
Cisplatin is a nephrotoxic chemotherapeutic agent. So, preventive measures worth to be evaluated. Human amniotic fluid stem cells (hAFSCs) in prevention or amelioration of cisplatin-induced acute kidney injury (AKI) in Sprague-Dawley rates have been tested.
METHODS
80 Sprague-Dawley rats (250~300 g) were used and divided into 4 major groups, 20 rats each. Group I: Saline-injected group. Group II: Cisplatin-injected group (5 mg/kg I.P). Group III: Cisplatin-injected and hAFSCs-treated group (5×106 hAFSCs I.V. one day after cisplatin administration). Group IV: Cisplatin-injected and culture media-treated group. Each major group was further divided into 4 equal subgroups according to the timing of sacrifice; 4, 7, 11 and 30 days post-cisplatin injection. Renal function tests were done. Kidney tissue homogenate oxidative stress parameters malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione (GSH) were determined. Histopathological scoring systems for active injury, regenerative and chronic changes were analyzed separately.
RESULTS
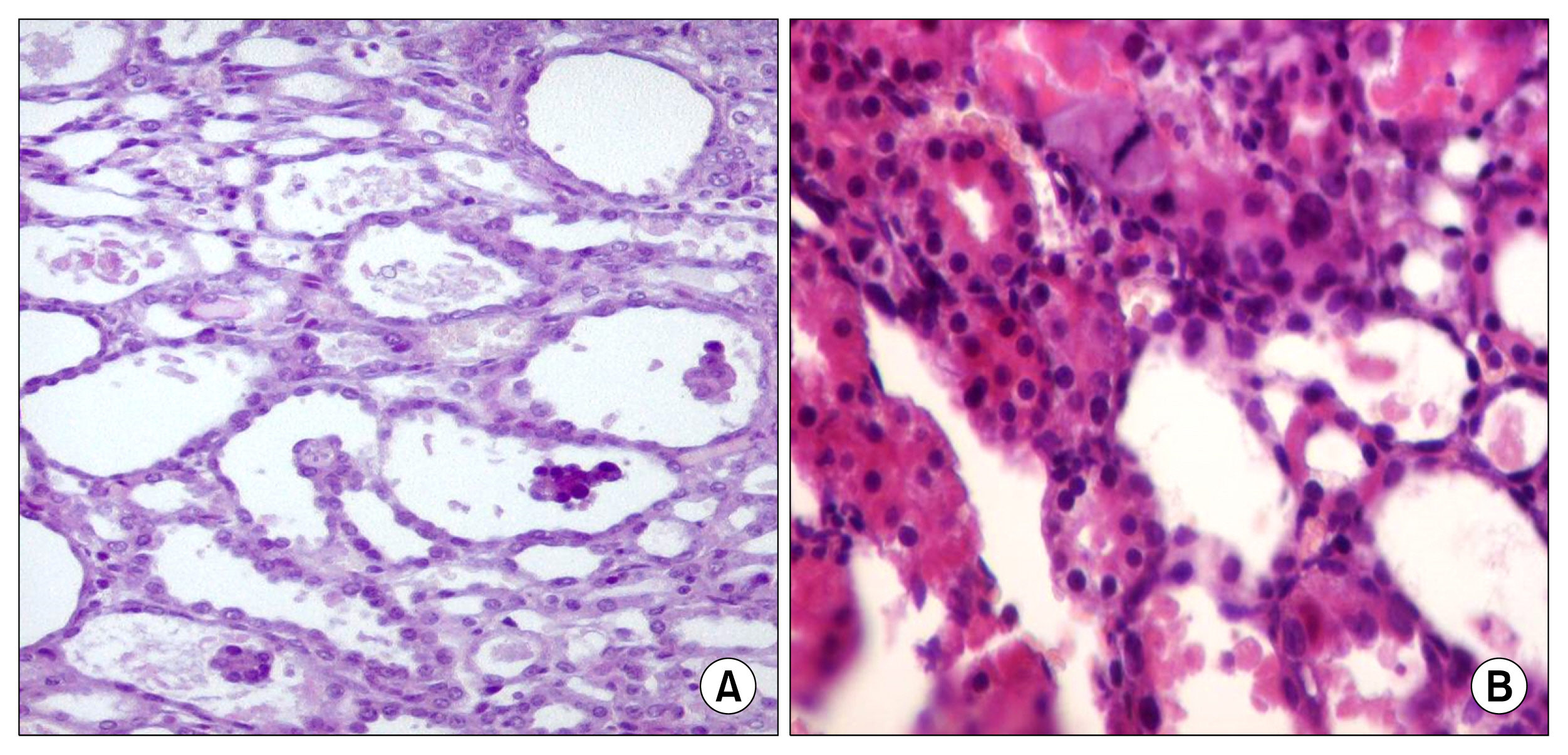
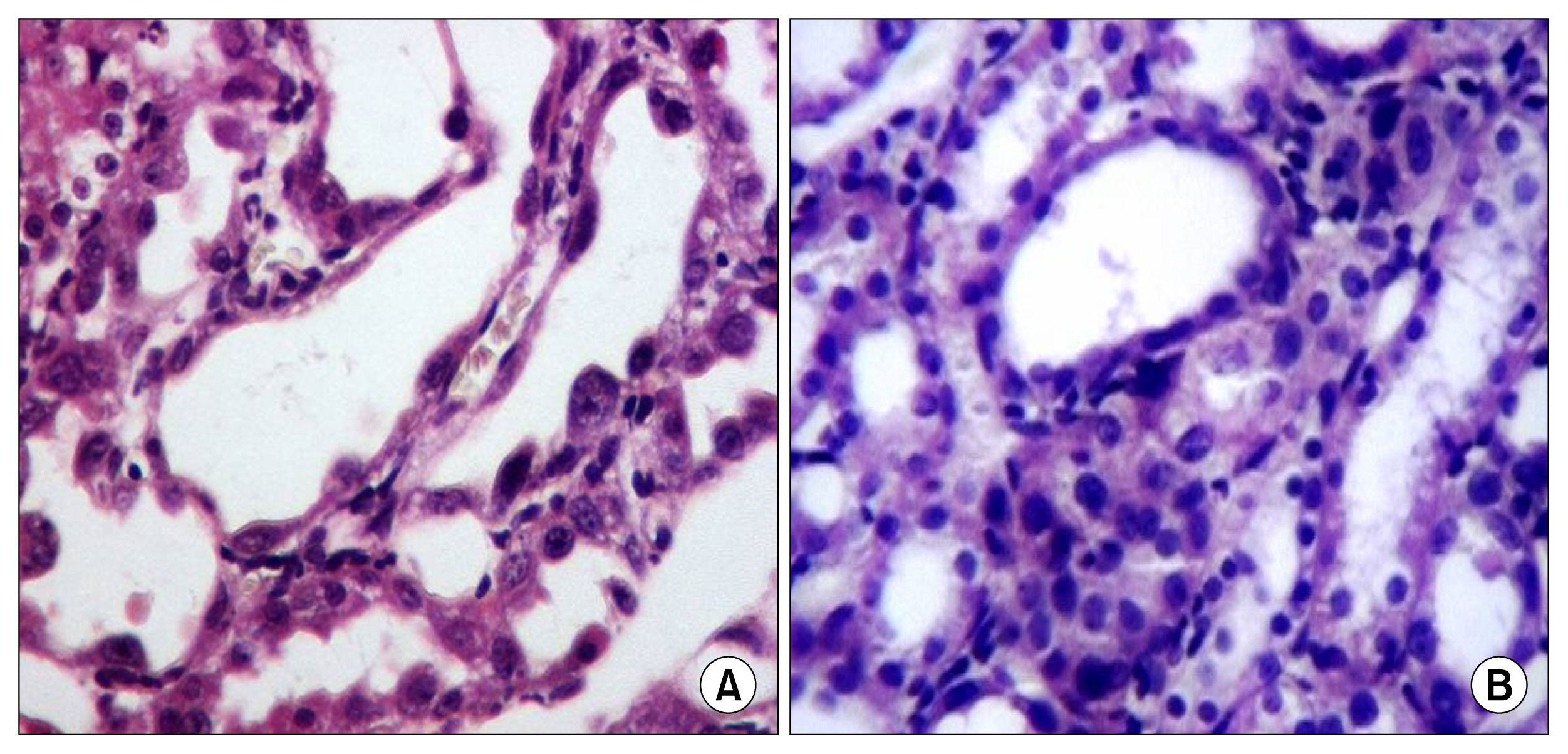
hAFSCs characterization and differentiation was proved. Cisplatin injection resulted in a significant increase in serum creatinine and MDA and decrease in SOD, GSH and creatinine clearance. These changes were attenuated early by day 4 with the use of hAFSCs. Cisplatin injection induced tubular necrosis, atrophy, inflammatory cells infiltration and fibrosis. The use of hAFSCs was associated with significantly lowered injury score at day 4, 7, 11 and 30 with marked regenerative changes starting from day 4.
CONCLUSION
hAFSCs have both a protective and regenerative activities largely through an antioxidant activity. This activity cut short the acuteness of cisplatin nephrotoxicity.
MeSH Terms
Figure
Reference
-
References
1. Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012; 380:756–766. DOI: 10.1016/S0140-6736(11)61454-2. PMID: 22617274.
Article2. Bentley ML, Corwin HL, Dasta J. Drug-induced acute kidney injury in the critically ill adult: recognition and prevention strategies. Crit Care Med. 2010; 38(6 Suppl):S169–S174. DOI: 10.1097/CCM.0b013e3181de0c60. PMID: 20502171.
Article3. Ciarimboli G, Ludwig T, Lang D, Pavenstädt H, Koepsell H, Piechota HJ, Haier J, Jaehde U, Zisowsky J, Schlatter E. Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am J Pathol. 2005; 167:1477–1484. DOI: 10.1016/S0002-9440(10)61234-5. PMID: 16314463. PMCID: 1613191.
Article4. Wei Q, Dong G, Franklin J, Dong Z. The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int. 2007; 72:53–62. DOI: 10.1038/sj.ki.5002256. PMID: 17410096.
Article5. Jo SK, Cho WY, Sung SA, Kim HK, Won NH. MEK inhibitor, U0126, attenuates cisplatin-induced renal injury by decreasing inflammation and apoptosis. Kidney Int. 2005; 67:458–466. DOI: 10.1111/j.1523-1755.2005.67102.x. PMID: 15673293.
Article6. Lee S, Moon SO, Kim W, Sung MJ, Kim DH, Kang KP, Jang YB, Lee JE, Jang KY, Lee SY, Park SK. Protective role of L-2-oxothiazolidine-4-carboxylic acid in cisplatin-induced renal injury. Nephrol Dial Transplant. 2006; 21:2085–2095. DOI: 10.1093/ndt/gfl209. PMID: 16705027.
Article7. Ramesh G, Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest. 2002; 110:835–842. DOI: 10.1172/JCI200215606. PMID: 12235115. PMCID: 151130.
Article8. Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008; 73:994–1007. DOI: 10.1038/sj.ki.5002786. PMID: 18272962.
Article9. Takaori K, Yanagita M. Kidney regeneration and stem cells. Anat Rec (Hoboken). 2014; 297:129–136. DOI: 10.1002/ar.22801.
Article10. Shokeir AA, Harraz AM, El-Din AB. Tissue engineering and stem cells: basic principles and applications in urology. Int J Urol. 2010; 17:964–973. DOI: 10.1111/j.1442-2042.2010.02643.x. PMID: 20969644.
Article11. Cananzi M, De Coppi P. CD117+ amniotic fluid stem cells: state of the art and future perspectives. Organogenesis. 2012; 8:77–88. DOI: 10.4161/org.22426. PMID: 23037870. PMCID: 3527320.12. Siegel N, Rosner M, Hanneder M, Freilinger A, Hengstschläger M. Human amniotic fluid stem cells: a new perspective. Amino Acids. 2008; 35:291–293. DOI: 10.1007/s00726-007-0593-1.
Article13. Perin L, Giuliani S, Jin D, Sedrakyan S, Carraro G, Habibian R, Warburton D, Atala A, De Filippo RE. Renal differentiation of amniotic fluid stem cells. Cell Prolif. 2007; 40:936–948. DOI: 10.1111/j.1365-2184.2007.00478.x. PMID: 18021180.
Article14. Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003; 17:160–170. DOI: 10.1038/sj.leu.2402763. PMID: 12529674.
Article15. Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004; 103:1662–1668. DOI: 10.1182/blood-2003-09-3070.
Article16. van Roeyen CR, Ostendorf T, Denecke B, Bokemeyer D, Behrmann I, Strutz F, Lichenstein HS, LaRochelle WJ, Pena CE, Chaudhuri A, Floege J. Biological responses to PDGF-BB versus PDGF-DD in human mesangial cells. Kidney Int. 2006; 69:1393–1402. DOI: 10.1038/sj.ki.5000332. PMID: 16557224.
Article17. Saad SY, Arafah MM, Najjar TA. Effects of mycophenolate mofetil on cisplatin-induced renal dysfunction in rats. Cancer Chemother Pharmacol. 2007; 59:455–460. DOI: 10.1007/s00280-006-0284-8.
Article18. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358. DOI: 10.1016/0003-2697(79)90738-3. PMID: 36810.
Article19. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959; 82:70–77. DOI: 10.1016/0003-9861(59)90090-6. PMID: 13650640.
Article20. De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007; 25:100–106. DOI: 10.1038/nbt1274. PMID: 17206138.
Article21. Perin L, Sedrakyan S, Giuliani S, Da Sacco S, Carraro G, Shiri L, Lemley KV, Rosol M, Wu S, Atala A, Warburton D, De Filippo RE. Protective effect of human amniotic fluid stem cells in an immunodeficient mouse model of acute tubular necrosis. PLoS One. 2010; 5:e9357. DOI: 10.1371/journal.pone.0009357. PMID: 20195358. PMCID: 2827539.
Article22. Rota C, Imberti B, Pozzobon M, Piccoli M, De Coppi P, Atala A, Gagliardini E, Xinaris C, Benedetti V, Fabricio AS, Squarcina E, Abbate M, Benigni A, Remuzzi G, Morigi M. Human amniotic fluid stem cell preconditioning improves their regenerative potential. Stem Cells Dev. 2012; 21:1911–1923. DOI: 10.1089/scd.2011.0333. PMCID: 3396139.
Article23. Atessahin A, Yilmaz S, Karahan I, Ceribasi AO, Karaoglu A. Effects of lycopene against cisplatin-induced nephrotoxicity and oxidative stress in rats. Toxicology. 2005; 212:116–123. DOI: 10.1016/j.tox.2005.04.016. PMID: 15946783.
Article24. Kuhad A, Pilkhwal S, Sharma S, Tirkey N, Chopra K. Effect of curcumin on inflammation and oxidative stress in cisplatin-induced experimental nephrotoxicity. J Agric Food Chem. 2007; 55:10150–10155. DOI: 10.1021/jf0723965. PMID: 18001039.
Article25. Kim WS, Park BS, Kim HK, Park JS, Kim KJ, Choi JS, Chung SJ, Kim DD, Sung JH. Evidence supporting anti-oxidant action of adipose-derived stem cells: protection of human dermal fibroblasts from oxidative stress. J Dermatol Sci. 2008; 49:133–142. DOI: 10.1016/j.jdermsci.2007.08.004.
Article26. Vickers AE, Rose K, Fisher R, Saulnier M, Sahota P, Bentley P. Kidney slices of human and rat to characterize cisplatin-induced injury on cellular pathways and morphology. Toxicol Pathol. 2004; 32:577–590. DOI: 10.1080/01926230490508821. PMID: 15603542.
Article27. Imberti B, Morigi M, Tomasoni S, Rota C, Corna D, Longaretti L, Rottoli D, Valsecchi F, Benigni A, Wang J, Abbate M, Zoja C, Remuzzi G. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol. 2007; 18:2921–2928. DOI: 10.1681/ASN.2006121318. PMID: 17942965.28. Morigi M, Rota C, Montemurro T, Montelatici E, Lo Cicero V, Imberti B, Abbate M, Zoja C, Cassis P, Longaretti L, Rebulla P, Introna M, Capelli C, Benigni A, Remuzzi G, Lazzari L. Life-sparing effect of human cord blood-mesenchymal stem cells in experimental acute kidney injury. Stem Cells. 2010; 28:513–522. PMID: 20049901.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Study of the Effect of Route of Administration of Mesenchymal Stem Cells on Cisplatin-Induced Acute Kidney Injury in Sprague Dawley Rats
- Effect of Amniotic Fluid Stem Cells and Amniotic Fluid Cells on the Wound Healing Process in a White Rat Model
- Stem Cell Therapy for Erectile Dysfunction
- The Expressions of iNOS and the Effects of iNOS Inhibitor in the Rats of Cisplatin Induced Nephrotoxicity
- A Mini Overview of Isolation, Characterization and Application of Amniotic Fluid Stem Cells