Restor Dent Endod.
2016 May;41(2):91-97. 10.5395/rde.2016.41.2.91.
Antifungal effects of synthetic human β-defensin 3-C15 peptide
- Affiliations
-
- 1Department of Conservative Dentistry, Seoul National University Dental Hospital, Seoul National University School of Dentistry and Dental Research Institute, Seoul, Korea. kum6139@snu.ac.kr
- 2Department of Oral Microbiology and Immunology, DRI and BK21 Plus Program, Seoul National University School of Dentistry, Seoul, Korea.
- 3Department of Anatomy and Cell Biology, McGill University, Montreal, QC, Canada.
- 4Department of Biology, Siena College, Loudonville, NY, USA.
- 5Schulich School of Medicine and Dentistry, University of Western Ontario, London, ON, Canada.
- 6Department of Conservative Dentistry, School of Dentistry, Kyung Hee University, Seoul, Korea.
- 7Division of Endodontology, Department of Oral Health and Diagnostic Sciences, University of Connecticut Health Center, School of Dental Medicine, Farmington, CT, USA.
- KMID: 2163290
- DOI: http://doi.org/10.5395/rde.2016.41.2.91
Abstract
OBJECTIVES
The purpose of this ex vivo study was to compare the antifungal activity of a synthetic peptide consisting of 15 amino acids at the C-terminus of human β-defensin 3 (HBD3-C15) with calcium hydroxide (CH) and Nystatin (Nys) against Candida albicans (C. albicans) biofilm.
MATERIALS AND METHODS
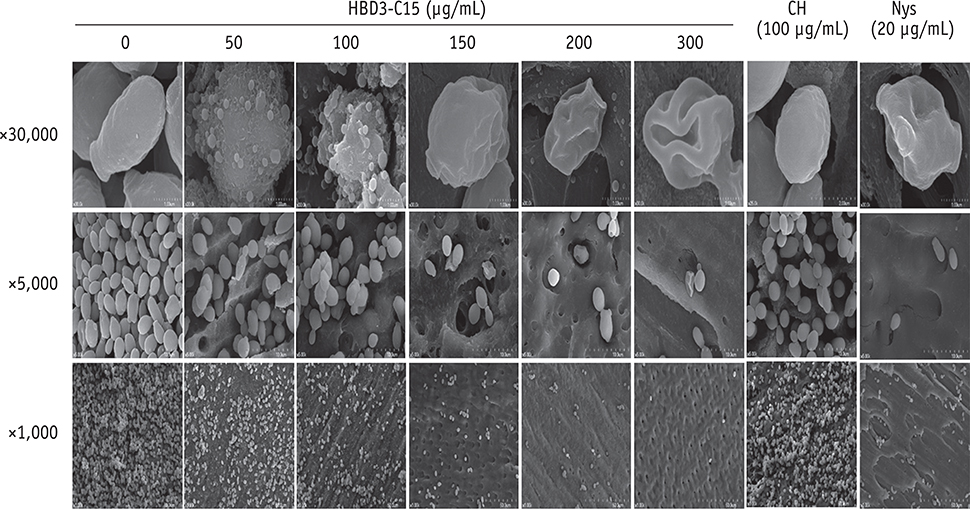
C. albicans were grown on cover glass bottom dishes or human dentin disks for 48 hr, and then treated with HBD3-C15 (0, 12.5, 25, 50, 100, 150, 200, and 300 µg/mL), CH (100 µg/mL), and Nys (20 µg/mL) for 7 days at 37℃. On cover glass, live and dead cells in the biomass were measured by the FilmTracer Biofilm viability assay, and observed by confocal laser scanning microscopy (CLSM). On dentin, normal, diminished and ruptured cells were observed by field-emission scanning electron microscopy (FE-SEM). The results were subjected to a two-tailed t-test, a one way analysis variance and a post hoc test at a significance level of p = 0.05.
RESULTS
C. albicans survival on dentin was inhibited by HBD3-C15 in a dose-dependent manner. There were fewer aggregations of C. albicans in the groups of Nys and HBD3-C15 (≥ 100 µg/mL). CLSM showed C. albicans survival was reduced by HBD3-C15 in a dose dependent manner. Nys and HBD3-C15 (≥ 100 µg/mL) showed significant fungicidal activity compared to CH group (p < 0.05).
CONCLUSIONS
Synthetic HBD3-C15 peptide (≥ 100 µg/mL) and Nys exhibited significantly higher antifungal activity than CH against C. albicans by inhibiting cell survival and biofilm.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Endodontic biofilms: contemporary and future treatment options
Yeon-Jee Yoo, Hiran Perinpanayagam, Soram Oh, A-Reum Kim, Seung-Hyun Han, Kee-Yeon Kum
Restor Dent Endod. 2019;44(1):. doi: 10.5395/rde.2019.44.e7.
Reference
-
1. Waltimo TMT, Haapasalo M, Zehnder M, Meyer J. Clinical aspects related to endodontic yeast infections. Endod Topics. 2004; 9:66–78.
Article2. Naglik JR, Rodgers CA, Shirlaw PJ, Dobbie JL, Fernandes-Naglik LL, Greenspan D, Agabian N, Challacombe SJ. Differential expression of Candida albicans secreted aspartyl proteinase and phospholipase B genes in humans correlates with active oral and vaginal infections. J Infect Dis. 2003; 188:469–479.
Article3. Siqueira JF Jr, Rôças IN, Lopes HP, Elias CN, de Uzeda M. Fungal infection of the radicular dentin. J Endod. 2002; 28:770–773.
Article4. Mitchell KF, Zarnowski R, Sancheza H, Edward JA, Reinicke EL, Nett JE, Mitchell AP, Andes DR. Community participation in biofilm matrix assembly and function. Proc Natl Acad Sci U S A. 2015; 112:4092–4097.
Article5. Kumamoto CA. Candida biofilms. Curr Opin Microbiol. 2002; 5:608–611.
Article6. Mah TF, O'Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001; 9:34–39.
Article7. Sundqvist G, Figdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative retreatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998; 85:86–93.
Article8. Nair PN, Sjögren U, Krey G, Kahnberg KE, Sundqvist G. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical Lesions: a long-term light and electron microscopic follow-up study. J Endod. 1990; 16:580–588.
Article9. Byström A, Sundqvist G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res. 1981; 89:321–328.
Article10. Gupta SP, Bhati M, Jhajharia K, Patel H, Paliwal A, Franklin S. Evaluation of antimicrobial and antifungal efficacy of inter appointment intracanal medicaments against Enterococcus and Candida albicans: an in vitro study. J Int Oral Health. 2015; 7:97–102.11. Law A, Messer H. An evidence-based analysis of the antibacterial effectiveness of intracanal medicaments. J Endod. 2004; 30:689–694.
Article12. Siqueira JF Jr, Sen BH. Fungi in endodontic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004; 97:632–641.
Article13. Rosato A, Vitali C, Piarulli M, Mazzotta M, Argentieri MP, Mallamaci R. In vitro synergic efficacy of the combination of Nystatin with the essential oils of Origanum vulgare and Pelargonium graveolens against some Candida species. Phytomedicine. 2009; 16:972–975.
Article14. Kaomongkolgit R, Jamdee K, Chaisomboon N. Antifungal activity of alpha-mangostin against Candida albicans. J Oral Sci. 2009; 51:401–406.
Article15. Lima SM, de Pádua GM, Sousa MG, Freire Mde S, Franco OL, Rezende TM. Antimicrobial peptide-based treatment for endodontic infections-biotechnological innovation in endodontics. Biotechnol Adv. 2015; 33:203–213.
Article16. Paris S, Wolgin M, Kielbassa AM, Pries A, Zakrzewicz A. Gene expression of human beta-defensins in healthy and inflamed human dental pulps. J Endod. 2009; 35:520–523.
Article17. Schibli DJ, Hunter HN, Aseyev V, Starner TD, Wiencek JM, McCray PB Jr, Tack BF, Vogel HJ. The solution structures of the human beta-defensins lead to a better understanding of the potent bactericidal activity of HBD3 against Staphylococcus aureus. J Biol Chem. 2002; 277:8279–8289.
Article18. Dhople V, Krukemeyer A, Ramamoorthy A. The human beta-defensin-3, an antibacterial peptide with multiple biological functions. Biochim Biophys Acta. 2006; 1758:1499–1512.
Article19. Lee JK, Park YJ, Kum KY, Han SH, Chang SW, Kaufman B, Jiang J, Zhu Q, Safavi K, Spångberg L. Antimicrobial efficacy of a human beta-defensin-3 peptide using an Enterococcus faecalis dentine infection model. Int Endod J. 2013; 46:406–412.
Article20. Lee JK, Chang SW, Perinpanayagam H, Lim SM, Park YJ, Han SH, Baek SH, Zhu Q, Bae KS, Kum KY. Antibacterial efficacy of a human beta-defensin-3 peptide on multispecies biofilms. J Endod. 2013; 39:1625–1629.
Article21. Krishnakumari V, Rangaraj N, Nagaraj R. Antifungal activities of human beta-defensins HBD-1 to HBD-3 and their C-terminal analogs Phd1 to Phd3. Antimicrob Agents Chemother. 2009; 53:256–260.
Article22. Hoover DM, Wu Z, Tucker K, Lu W, Lubkowski J. Antimicrobial characterization of human beta-defensin 3 derivatives. Antimicrob Agents Chemother. 2003; 47:2804–2809.
Article23. Song W, Shi Y, Xiao M, Lu H, Qu T, Li P, Wu G, Tian Y. In vitro bactericidal activity of recombinant human beta-defensin-3 against pathogenic bacterial strains in human tooth root canal. Int J Antimicrob Agents. 2009; 33:237–243.
Article24. Taff HT, Nett JE, Andes DR. Comparative analysis of Candida biofilm quantitation assays. Med Mycol. 2012; 50:214–218.
Article25. Guerreiro-Tanomaru JM, de Faria-Júnior NB, Duarte MA, Ordinola-Zapata R, Graeff MS, Tanomaru-Filho M. Comparative analysis of Enterococcus faecalis biofilm formation on different substrates. J Endod. 2013; 39:346–350.
Article26. Kim D, Kim E. Antimicrobial effect of calcium hydroxide as an intracanal medicament in root canal treatment: a literature review - Part II. in vivo studies. Restor Dent Endod. 2015; 40:97–103.
Article27. Mohammadi Z, Shalavi S, Yazdizadeh M. Antimicrobial activity of calcium hydroxide in endodontics: a review. Chonnam Med J. 2012; 48:133–140.
Article28. Wang JD, Hume WR. Diffusion of hydrogen ion and hydroxyl ion from various sources through dentine. Int Endod J. 1988; 21:17–26.
Article29. Kinsky SC. Nystatin binding by protoplasts and a particulate fraction of Neurospora crassa, and a basis for the selective toxicity of polyene antifungal antibiotics. Proc Natl Acad Sci U S A. 1962; 48:1049–1056.
Article30. de Kruijff BD, Demel RA. Polyene antibiotic-sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. III. Molecular structure of the polyene antibiotic-cholesterol complexes. Biochim Biophys Acta. 1974; 339:57–70.
Article31. Mohammadi Z. Systemic, prophylactic and local applications of antimicrobials in endodontics: an update review. Int Dent J. 2009; 59:175–186.32. Vylkova S, Li XS, Berner JC, Edgerton M. Distinct antifungal mechanisms: beta-defensins require Candida albicans Ssa1 protein, while Trk1p mediates activity of cysteine-free cationic peptides. Antimicrob Agents Chemother. 2006; 50:324–331.
Article33. Feng Z, Jiang B, Chandra J, Ghannoum M, Nelson S, Weinberg A. Human beta-defensins: differential activity against Candidal species and regulation by Candida albicans. J Dent Res. 2005; 84:445–450.
Article34. Klüver E, Schulz-Maronde S, Scheid S, Meyer B, Forssmann WG, Adermann K. Structure-activity relation of human β-defensin 3: influence of disulfide bonds and cysteine substitution on antimicrobial activity and cytotoxicity. Biochemistry. 2005; 44:9804–9816.
Article35. Maisetta G, Batoni G, Esin S, Luperini F, Pardini M, Bottai D, Florio W, Giuca MR, Gabriele M, Campa M. Activity of human β-defensin 3 alone or combined with other antimicrobial agents against oral bacteria. Antimicrob Agents Chemother. 2003; 47:3349–3351.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Antimicrobial Defensin Peptides of the Human Nasal Mucosa
- Ultrastructural immunolocalization of beta-defensin-27 in granulocytes of the dermis and wound epidermis of lizard suggests they contribute to the anti-microbial skin barrier
- The effects of newly formed synthetic peptide on bone regeneration in rat calvarial defects
- Regulation of Human Beta-Defensin 3(hBD-3) in Human Keratinocyte(HaCaT) Cell Lines
- Effects of Several Antifungal Agents on Cultured Human Nail Matrix Cells and Epidermal Keratinocytes