Anat Cell Biol.
2013 Dec;46(4):246-253. 10.5115/acb.2013.46.4.246.
Ultrastructural immunolocalization of beta-defensin-27 in granulocytes of the dermis and wound epidermis of lizard suggests they contribute to the anti-microbial skin barrier
- Affiliations
-
- 1Department of Bigea, University of Bologna, Bologna, Italy. lorenzo.alibardi@unibo.it
- KMID: 2046765
- DOI: http://doi.org/10.5115/acb.2013.46.4.246
Abstract
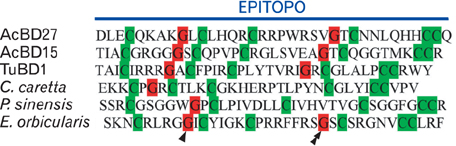
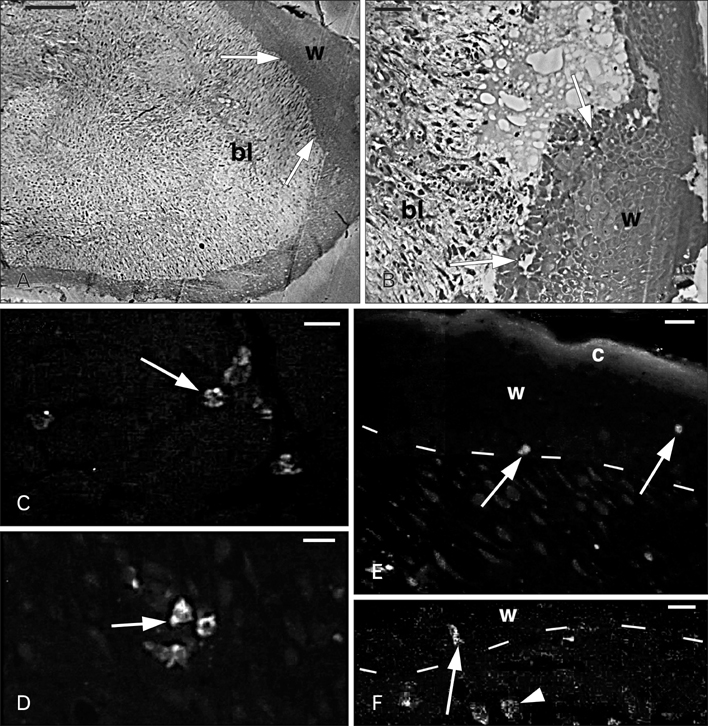
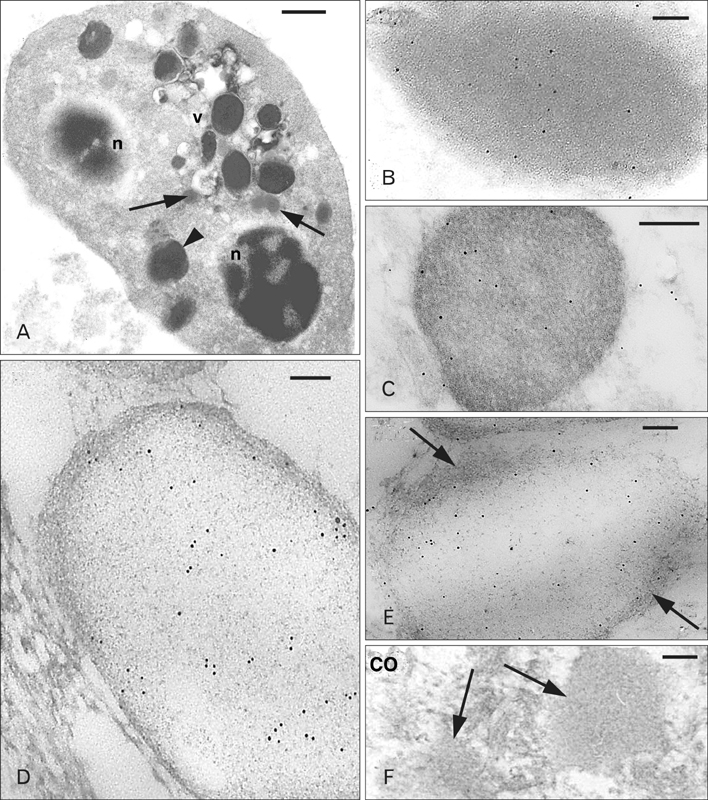
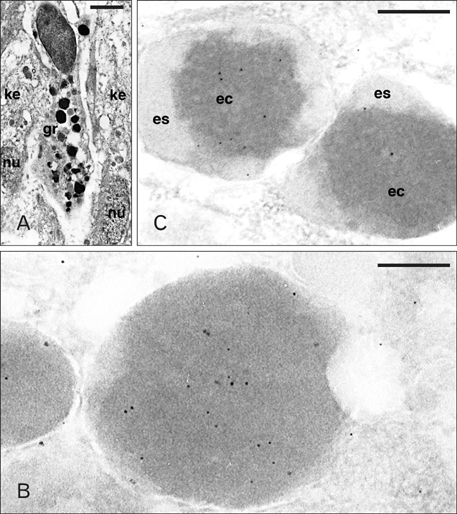
- The high resistance to infections in lizard wounds suggests that these reptiles possess effective antimicrobial peptides in their tissues. The present immunocytochemical study shows the cellular localization of beta-defensin 27 in tail tissues and in the blood, a defensin previously identified in the lizard Anolis carolinensis through biomolecular methods. Beta-defensin-27 immunoreactivity is only observed in some large granules mainly contained in heterophilic granulocytes that are sparse within the dermis of the skin or in the isolated blood. This peptide is absent in other cell types of the skin, in keratinocytes and in subdermal muscle tissue of the tail in normal conditions. Pre-corneous keratinocytes of the regenerating tail epidermis are unlabeled or show a weak labeling for the peptide only in sparse cytoplasmic areas or in the extracellular spaces among corneocytes of the wound and regenerating epidermis. The study suggests that beta-defensin 27 is normally stored in granulocytes present in the blood or in connective tissues while in the epidermis keratinocytes do not show the presence of this peptide unless these cells are stimulated from injury to produce and likely release beta-defensins.
Keyword
MeSH Terms
Figure
Reference
-
1. Cox PG. Some aspects of tail regeneration in the lizard Anolis carolinensis. I. A description based on histology and autoradiography. J Exp Zool. 1969; 171:127–149.2. Zika JM. A histological study of the regenerative response in a lizard, Anolis carolinensis. J Exp Zool. 1969; 172:1–8.3. Alibardi L. Ultrastructural features of the process of wound healing after tail and limb amputation in lizard. Acta Zool. 2010; 91:306–318.4. Alibardi L, Toni M. Wound keratins in the regenerating epidermis of lizard suggest that the wound reaction is similar in the tail and limb. J Exp Zool A Comp Exp Biol. 2005; 303:845–860.5. Alibardi L, Celeghin A, Dalla Valle L. Wounding in lizards results in the release of beta-defensins at the wound site and formation of an antimicrobial barrier. Dev Comp Immunol. 2012; 36:557–565.6. Dalla Valle L, Benato F, Maistro S, Quinzani S, Alibardi L. Bioinformatic and molecular characterization of beta-defensins-like peptides isolated from the green lizard Anolis carolinensis. Dev Comp Immunol. 2012; 36:222–229.7. Dalla Valle L, Benato F, Paccanaro MC, Alibardi L. Bioinformatic and molecular characterization of cathelicidin-like peptides isolated from the green lizard Anolis carolinensis (Reptilia: Lepidosauria: Iguanidae). Ital J Zool. 2013; 80:177–186.8. Sahl HG, Pag U, Bonness S, Wagner S, Antcheva N, Tossi A. Mammalian defensins: structures and mechanism of antibiotic activity. J Leukoc Biol. 2005; 77:466–475.9. Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005; 6:551–557.10. Chattopadhyay S, Sinha NK, Banerjee S, Roy D, Chattopadhyay D, Roy S. Small cationic protein from a marine turtle has beta-defensin-like fold and antibacterial and antiviral activity. Proteins. 2006; 64:524–531.11. Lakshminarayanan R, Vivekanandan S, Samy RP, Banerjee Y, Chi-Jin EO, Teo KW, Jois SD, Kini RM, Valiyaveettil S. Structure, self-assembly, and dual role of a beta-defensin-like peptide from the Chinese soft-shelled turtle eggshell matrix. J Am Chem Soc. 2008; 130:4660–4668.12. Stegemann C, Kolobov A Jr, Leonova YF, Knappe D, Shamova O, Ovchinnikova TV, Kokryakov VN, Hoffmann R. Isolation, purification and de novo sequencing of TBD-1, the first beta-defensin from leukocytes of reptiles. Proteomics. 2009; 9:1364–1373.13. Benato F, Dalla Valle L, Skobo T, Alibardi L. Biomolecular identification of beta-defensin-like peptides from the skin of the soft-shelled turtle Apalone spinifera. J Exp Zool B Mol Dev Evol. 2013; 320:210–217.14. Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004; 75:39–48.15. Zelezetsky I, Pontillo A, Puzzi L, Antcheva N, Segat L, Pacor S, Crovella S, Tossi A. Evolution of the primate cathelicidin. Correlation between structural variations and antimicrobial activity. J Biol Chem. 2006; 281:19861–19871.16. Panyutich A, Shi J, Boutz PL, Zhao C, Ganz T. Porcine polymorphonuclear leukocytes generate extracellular microbicidal activity by elastase-mediated activation of secreted proprotegrins. Infect Immun. 1997; 65:978–985.17. Alibardi L. Granulocytes of reptiles contain Beta-defensin.like peptides: a comparative ultrastructural survey. J Morphol. 2013; 274:877–886.18. Evans EW, Beach GG, Wunderlich J, Harmon BG. Isolation of antimicrobial peptides from avian heterophils. J Leukoc Biol. 1994; 56:661–665.19. Stolzenberg ED, Anderson GM, Ackermann MR, Whitlock RH, Zasloff M. Epithelial antibiotic induced in states of disease. Proc Natl Acad Sci U S A. 1997; 94:8686–8690.20. Oren A, Ganz T, Liu L, Meerloo T. In human epidermis, beta-defensin 2 is packaged in lamellar bodies. Exp Mol Pathol. 2003; 74:180–182.21. Braff MH, Di Nardo A, Gallo RL. Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J Invest Dermatol. 2005; 124:394–400.22. Menzies BE, Kenoyer A. Staphylococcus aureus infection of epidermal keratinocytes promotes expression of innate antimicrobial peptides. Infect Immun. 2005; 73:5241–5244.23. Rivas-Santiago B, Schwander SK, Sarabia C, Diamond G, Klein-Patel ME, Hernandez-Pando R, Ellner JJ, Sada E. Human {beta}-defensin 2 is expressed and associated with Mycobacterium tuberculosis during infection of human alveolar epithelial cells. Infect Immun. 2005; 73:4505–4511.24. Dalla Valle L, Benato F, Maistro S, Quinzani S, Alibardi L. Bioinformatic and molecular characterization of beta-defensins-like peptides isolated from the green lizard Anolis carolinensis. Dev Comp Immunol. 2011; 36:222–229.25. van Dijk A, Molhoek EM, Bikker FJ, Yu PL, Veldhuizen EJ, Haagsman HP. Avian cathelicidins: paradigms for the development of anti-infectives. Vet Microbiol. 2011; 153:27–36.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical Study of Human beta Defensin-2 Expression in Superficial Mycosis
- Maximal Tension of Human Epidermis Prepared from Suction Blisters
- Expression of Human beta-defensin mRNA in Epidermal Proliferative Diseases
- The Expression of beta-defensin 1 in Various Skin Tumors
- Hydrolysis of Adenylic Acid by Human Skin Tissue in vitro: 5'-Nucleotidase Activity in the Epidermis and Dermis