Yonsei Med J.
2008 Oct;49(5):819-827. 10.3349/ymj.2008.49.5.819.
Use of Long-term Cultured Embryoid Bodies May Enhance Cardiomyocyte Differentiation by BMP2
- Affiliations
-
- 1Institute of Reproductive Medicine and Population, Medical Research Center, Seoul, Korea. ymchoi@snu.ac.kr
- 2Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2158201
- DOI: http://doi.org/10.3349/ymj.2008.49.5.819
Abstract
- PURPOSE
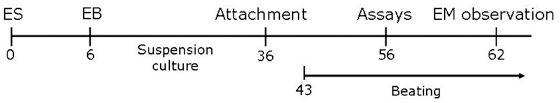
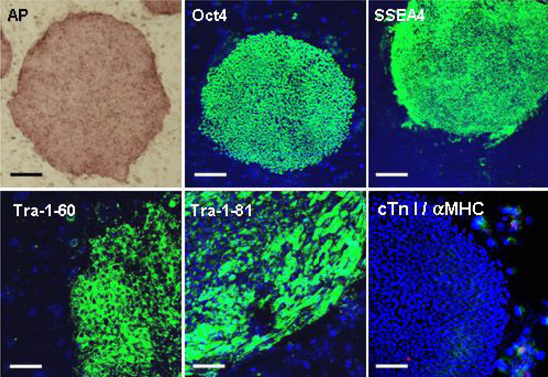
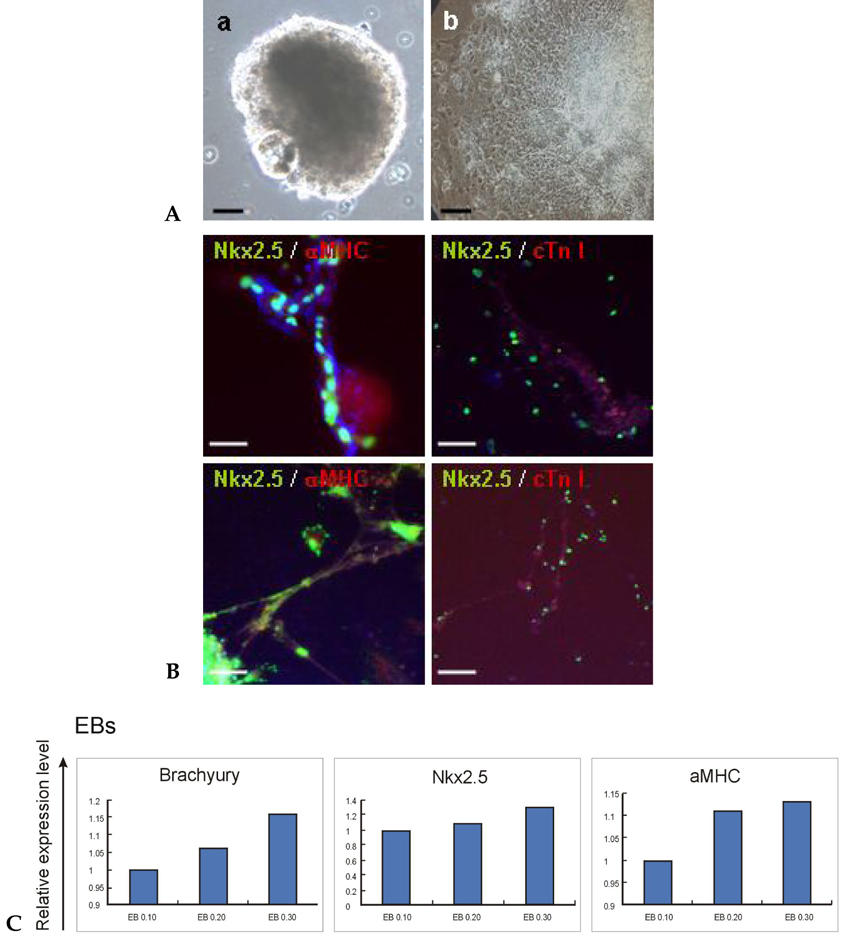
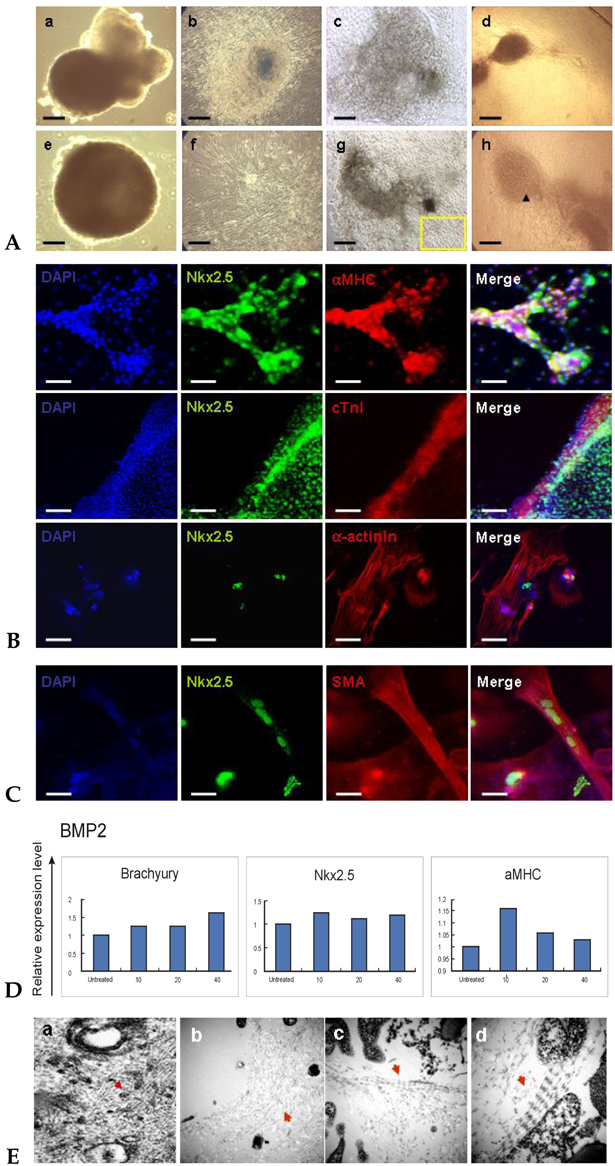
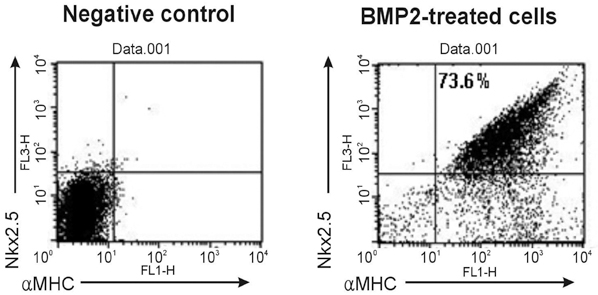
Human embryonic stem cells (hESCs) can proliferate for a prolonged period and differentiate into cardiomyocytes in vitro. Recent studies used bone morphogenetic protein 2 (BMP2) to generate cardiomyocytes from hESCs, however, all those studies used early embryoid bodies (EBs) and did not retrieve cardiomyocytes with a high yield. In this study, we treated long-term cultured EBs with BMP2 in order to promote differentiation into cardiomyocytes from hESCs. MATERIALS AND METHODS: hESC lines, including SNUhES3 and SNUhES4, were used in this study. Undifferentiated hESC colonies were detached to form EBs and cultured for up to 30 days. These long-term cultured EBs were differentiated into cardiomyocytes in serum-containing media. In our protocol, BMP2 was applied for 5 days after attachment of EBs. Cardiac specific markers, beating of differentiated cells and electron microscopic (EM) ultrastructures were evaluated and analyzed. RESULTS: Compared to 10-day or 20-day EBs, 30-day EBs showed a higher expression level of cardiac specific markers, Nkx2.5 and a-myosin heavy chain (alphaMHC). Treatment of BMP2 increased expression of cardiac troponin (cTn) I and a-actinin when evaluated at 20 days after attachment of 30-day EBs. Beating of differentiated cells was observed from 7 to 20 days after attachment. Moreover, EM findings demonstrated fine structures such as Z bands in these differentiated cardiomyocytes. These long-term cultured EBs yielded cardiomyocytes with an efficiency of as high as 73.6% when assessed by FACS. CONCLUSION: We demonstrated that the use of long-term cultured EBs may enhance differentiation into cardiomyocytes from hESCs when treated with BMP2.
Keyword
MeSH Terms
Figure
Reference
-
1. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998. 282:1145–1147.
Article2. Oh SK, Kim HS, Ahn HJ, Seol HW, Kim YY, Park YB, et al. Derivation and characterization of new human embryonic stem cell lines: SNUhES1, SNUhES2 and SNUhES3. Stem Cells. 2005. 23:211–219.
Article3. Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001. 108:407–414.
Article4. Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002. 91:501–508.
Article5. Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003. 107:2733–2740.
Article6. He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003. 93:32–39.7. Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004. 22:1282–1289.
Article8. Passier R, Oostwaard DW, Snapper J, Kloots J, Hassink R, Kuijk E, et al. Increased cardiomyocyte differentiation from human embryonic stem cells in serum-free cultures. Stem Cells. 2005. 23:772–780.9. Beqqali A, Kloots J, Ward-van Oostwaard D, Mummery C, Passier R. Genome-wide transcriptional profiling of human embryonic stem cells differentiating to cardiomyocytes. Stem Cells. 2006. 24:1956–1967.
Article10. Bettiol E, Sartiani L, Chicha L, Krause KH, Cerbai E, Jaconi ME. Fetal bovine serum enables cardiac differentiation of human embryonic stem cells. Differentiation. 2007. 75:669–681.
Article11. Schlange T, Andrée B, Arnold HH, Brand T. BMP2 is required for early heart development during a distinct time period. Mech Dev. 2000. 91:259–270.
Article12. Yuasa S, Itabashi Y, Koshimizu U, Tanaka T, Sugimura K, Kinoshita M, et al. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nature Biotechnol. 2005. 23:607–611.13. Antin PB, Taylor RG, Yatskievych T. Precardiac mesoderm is specified during gastrulation in quail. Dev Dyn. 1994. 200:144–154.
Article14. Schultheiss TM, Burch JB, Lassar AB. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev. 1997. 11:451–462.
Article15. Tomescot A, Leschik J, Bellamy V, Dubois G, Messas E, Bruneval P, et al. Differentiation in vivo of cardiac committed human embryonic stem cells in postmyocardial infarcted rats. Stem Cells. 2007. 25:2200–2205.
Article16. Pal R, Khanna A. Similar pattern in cardiac differentiation of human embryonic stem cell lines, BG01V and ReiCell® hES1, under low serum concentration supplemented with bone morphogenetic protein-2. Differentiation. 2007. 75:112–122.
Article17. Kwon YD, Oh SK, Kim HS, Ku SY, Kim SH, Choi YM, et al. Cellular manipulation of human embryonic stem cells by TAT-PDX1 protein transduction. Mol Ther. 2005. 12:28–32.
Article18. Oh SK, Kim HS, Park YB, Seol HW, Kim YY, Cho MS, et al. Methods for expansion of human embryonic stem cells. Stem Cells. 2005. 23:605–609.
Article19. Garry DJ, Olson EN. A common progenitor at the heart of development. Cell. 2006. 127:1101–1104.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effects of BMP4 and BMP2 on Expression of Trophoblast-Specific Genes in Human Embryonic Stem Cells using SNU-hES3 cell line
- Studies on Differentiation of Cardiac Myoblast Induced by Co-culture with Isolated Neonatal Rat Cardiac Myocytes
- Assessment of Developmental Toxicants using Human Embryonic Stem Cells
- SIRT1 Inhibits p53 but not NF-kappaB Transcriptional Activity during Differentiation of Mouse Embryonic Stem Cells into Embryoid Bodies
- Hematopoietic Differentiation of Embryoid Bodies Derived from the Human Embryonic Stem Cell Line SNUhES3 in Co-culture with Human Bone Marrow Stromal Cells