Yonsei Med J.
2008 Oct;49(5):804-810. 10.3349/ymj.2008.49.5.804.
The Expression of Adiponectin Receptors and the Effects of Adiponectin and Leptin on Airway Smooth Muscle Cells
- Affiliations
-
- 1Department of Pediatrics, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. jy7.shim@samsung.com
- 2Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2158199
- DOI: http://doi.org/10.3349/ymj.2008.49.5.804
Abstract
- PURPOSE
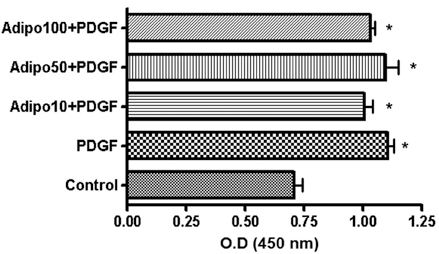
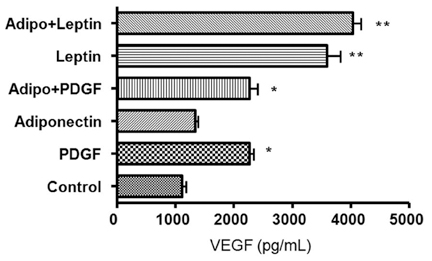
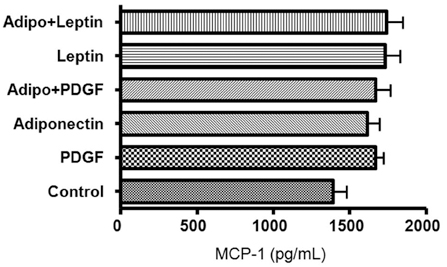
Obesity is a major risk factor for asthma and it influences airway smooth muscle function and responsiveness. Adiponectin is inversely associated with obesity and its action is mediated through at least 2 cell membrane receptors (AdipoR1 and AdipoR2). Leptin is positively associated with obesity. We investigated whether human airway smooth muscle (ASM) cells express adiponectin receptors and whether adiponectin and leptin regulate human ASM cell proliferation and vascular endothelial growth factor (VEGF) release. MATERIALS AND METHODS: Human ASM cells were growth-arrested in serum-deprived medium for 48 hours and then stimulated with PDGF, adiponectin and leptin. After 48 hours of stimulation, proliferation was determined using a cell proliferation ELISA kit. Human AdipoR1 and -R2 mRNA expressions were determined by RT-PCR using human-specific AdipoR1 and -R2 primers. Concentrations of VEGF, monocyte chemotactic protein (MCP)-1 and macrophage inflammatory protein (MIP)-1alpha in cell culture supernatant were determined by ELISA. RESULTS: Both AdipoR1 and AdipoR2 mRNA were expressed in the cultured human ASM cells. However, adiponectin did not suppress PDGF-enhanced ASM cell proliferation, nor did leptin promote ASM cell proliferation. Leptin promoted VEGF release by human ASM cells, while adiponectin did not influence VEGF release. Neither leptin nor adiponectin influenced MCP-1 secretion from human ASM cells. Adiponectin and MIP-1alpha were not secreted by human ASM cells. CONCLUSION: Human ASM cells expressed adiponectin receptors. However, adiponectin did not regulate human ASM cell proliferation or VEGF release, while leptin stimulated VEGF release by human ASM cells.
Keyword
MeSH Terms
-
Adiponectin/metabolism/*pharmacology/physiology
Cell Proliferation/*drug effects
Cells, Cultured
Chemokine CCL2/metabolism
Chemokine CCL3/metabolism
Humans
Leptin/metabolism/*pharmacology/physiology
Myocytes, Smooth Muscle/cytology/drug effects/*metabolism
Obesity/metabolism
Platelet-Derived Growth Factor/metabolism
Receptors, Adiponectin/*metabolism
Respiratory System/cytology/metabolism
Vascular Endothelial Growth Factor A/metabolism
Figure
Cited by 2 articles
-
Peroxisome Proliferator-Activated Receptor-Gamma Expression in the Lung Tissue of Obese Rats
Su Jin Hwang, Jung Ho Kim, Jae Won Shim, Duk Soo Kim, Hye Lim Jung, Moon Soo Park, Won Young Lee, Se-Yeon Kim, Jung Yeon Shim
Yonsei Med J. 2011;52(3):495-501. doi: 10.3349/ymj.2011.52.3.495.Effects of Diet-Induced Mild Obesity on Airway Hyperreactivity and Lung Inflammation in Mice
Sun Hee Jung, Jang-Mi Kwon, Jae Won Shim, Deok Soo Kim, Hye Lim Jung, Moon Soo Park, Soo-Hee Park, Jinmi Lee, Won-Young Lee, Jung Yeon Shim
Yonsei Med J. 2013;54(6):1430-1437. doi: 10.3349/ymj.2013.54.6.1430.
Reference
-
1. Castro-Rodríguez JA, Holberg CJ, Morgan WJ, Wright AL, Martinez FD. Increased incidence of asthmalike symptoms in girls who become overweight or obese during the school years. Am J Respir Crit Care Med. 2001. 163:1344–1349.
Article2. Hakala K, Stenius-Aarniala B, Sovijärvi A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest. 2000. 118:1315–1321.
Article3. Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005. 115:103–109.
Article4. Litonjua AA, Sparrow D, Celedon JC, DeMolles D, Weiss ST. Association of body mass index with the development of methacholine airway hyperresponsiveness in men: the normative aging study. Thorax. 2002. 57:581–585.
Article5. Shore SA, Rivera-Sanchez YM, Schwartzman IN, Johnston RA. Responses to ozone are increased in obese mice. J Appl Physiol. 2003. 95:938–945.
Article6. Shore SA, Fredberg JJ. Obesity, smooth muscle, and airway hyperresponsiveness. J Allergy Clin Immunol. 2005. 115:925–927.
Article7. Nagel G, Rapp K, Wabitsch M, Büchele G, Kroke A, Zöllner I, et al. Prevalence and cluster of cardiometabolic biomarkers in overweight and obese schoolchildren: results from a large survey in southwest Germany. Clin Chem. 2008. 54:317–325.
Article8. Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003. 52:1779–1785.9. Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003. 14:561–566.
Article10. Huang L, Li C. Leptin: a multifunctional hormone. Cell Res. 2000. 10:81–92.
Article11. Park HY, Kwon HM, Lim HJ, Hong BK, Lee JY, Park BE, et al. Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp Mol Med. 2001. 33:95–102.
Article12. Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003. 423:762–769.
Article13. Lee WY, Rhee EJ, Oh KW, Kim SY, Jung CH, Yun EJ, et al. Identification of adiponectin and its receptors in human osteoblast-like cells and association of T45G polymorphism in exon 2 of adiponectin gene with lumbar spine bone mineral density in Korean women. Clin Endocrinol (Oxf). 2006. 65:631–637.
Article14. Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006. 118:389–395.
Article15. Hersoug LG, Linneberg A. The link between the epidemics of obesity and allergic diseases: does obesity induce decreased immune tolerance? Allergy. 2007. 62:1205–1213.
Article16. Jin X, Fukuda N, Su J, Takagi H, Lai Y, Lin Z, et al. Effects of leptin on endothelial function with OB-Rb gene transfer in Zucker fatty rats. Atherosclerosis. 2003. 169:225–233.17. Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci U S A. 2001. 98:6390–6395.
Article18. Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007. 117:2362–2368.
Article19. Clauss M. Molecular biology of the VEGF and the VEGF receptor family. Semin Thromb Hemost. 2000. 26:561–569.
Article20. Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem. 1998. 273:13313–13316.
Article21. Nishigaki Y, Fujiuchi S, Yamazaki Y, Matsumoto H, Takeda A, Fujita Y, et al. Increased vascular endothelial growth factor in acute eosinophilic pneumonia. Eur Respir J. 2003. 21:774–778.
Article22. Meyer KC, Cardoni A, Xiang ZZ. Vascular endothelial growth factor in bronchoalveolar lavage from normal subjects and patients with diffuse parenchymal lung disease. J Lab Clin Med. 2000. 135:332–338.
Article23. Asai K, Kanazawa H, Otani K, Shiraishi S, Hirata K, Yoshikawa J. Imbalance between vascular endothelial growth factor and endostatin levels in induced sputum from asthmatic subjects. J Allergy Clin Immunol. 2002. 110:571–575.
Article24. Kanazawa H, Hirata K, Yoshikawa J. Involvement of vascular endothelial growth factor in exercise induced bronchoconstriction in asthmatic patients. Thorax. 2002. 57:885–888.
Article25. Sitaraman S, Liu X, Charrier L, Gu LH, Ziegler TR, Gewirtz A, et al. Colonic leptin: source of a novel proinflammatory cytokine involved in IBD. FASEB J. 2004. 18:696–698.
Article26. Murad A, Nath AK, Cha ST, Demir E, Flores-Riveros J, Sierra-Honigmann MR. Leptin is an autocrine/paracrine regulator of wound healing. FASEB J. 2003. 17:1895–1897.27. Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006. 116:1494–1505.
Article28. Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006. 116:115–124.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Leptin, Leptin Receptor, Adiponectin, and Adiponectin Receptor in Ductal Carcinoma In Situ and Invasive Breast Cancer
- Correlations of Leptin, Adiponectin and Leptin/Adiponectin Ratio with Metabolic Disorders in the Childhood Obesity
- The Effects of Adiponectin and Leptin in the Proliferation of Prostate Cancer Cells
- Puberty and Gender Differences of Plasma Leptin, Adiponectin Levels, and Leptin/Adiponectin Ratio
- The Effects of Antidepressants on the Leptin, Adiponectin, and Adiponectin Receptor mRNA Expression in Model Diabetogenic Rats