Yonsei Med J.
2007 Aug;48(4):653-658. 10.3349/ymj.2007.48.4.653.
High Concentrations of Pamidronate in Bone Weaken the Mechanical Properties of Intact Femora in a Rat Model
- Affiliations
-
- 1Department of Orthopaedic Surgery, Yongdong Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. kyang@yuhs.ac
- 2Central Research Institute, Hanlim Pharmaceutical Co., Ltd., Seoul, Korea.
- KMID: 2158171
- DOI: http://doi.org/10.3349/ymj.2007.48.4.653
Abstract
- PURPOSE
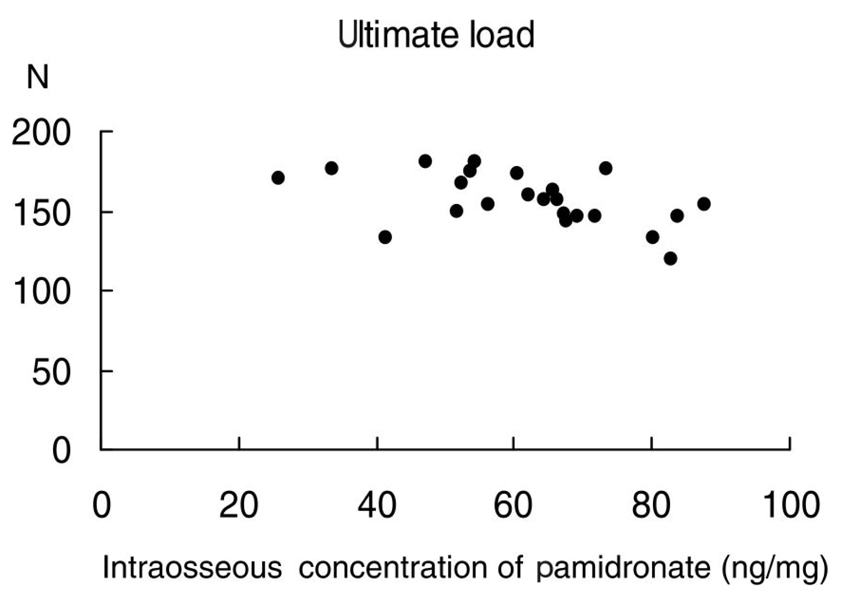
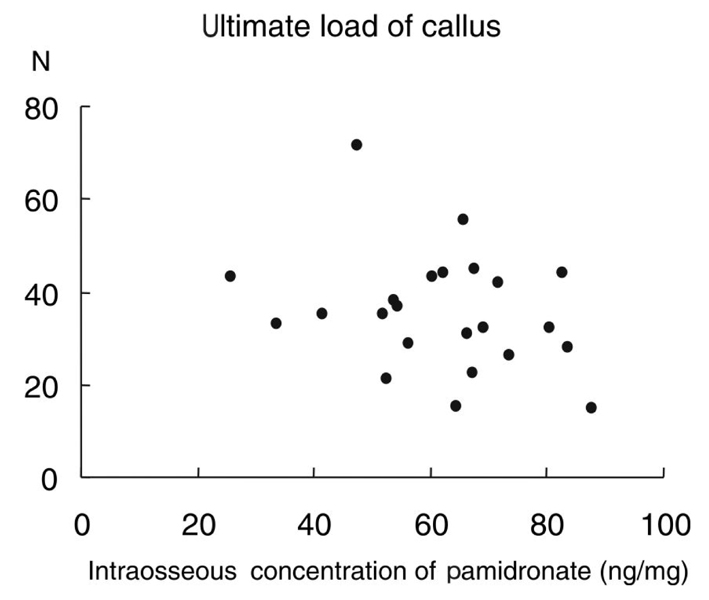
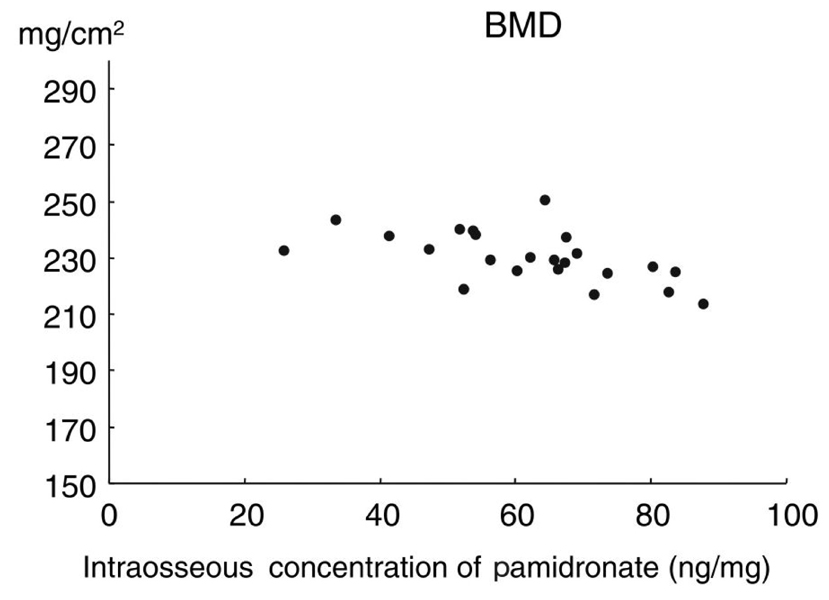
Bisphosphonates have been used to treat osteoporosis for more than ten years. However, complications associated with long-term administration of bisphosphonates, such as nonunion after pelvic insufficiency fracture or osteonecrosis of the jaw, have been recently reported in the literature. We investigated the relationships among the mechanical properties of the intact rat femur as well as healing fracture calluses and the intraosseous concentration of pamidronate (ICP), after long-term administration of pamidronate in a rat osteoporosis model. MATERIALS AND METHODS: We performed bilateral ovariectomy in 25 3-month-old female Sprague-Dawley rats. Beginning three months after ovariectomy, disodium pamidronate (0.5mg/kg) was injected every month. After the six-month administration period, the left femoral shaft was fractured using the closed fracture technique. Five weeks after fracture, 23 rats were euthanized and both femora were removed. We checked the mechanical properties of the intact (right) and fractured (left) femora using a three-point bending technique. Intraosseous concentration of pamidronate was checked by high-performance liquid chromatography. RESULTS: The mean ICP was 61.8+/-15.7ng/mg of bone. High ICP decreased the ultimate load to failure, stiffness, and ultimate stress of the intact femora (p=0.015, 0.027, 0.039, respectively). There was a tendency to decrease the ultimate load to failure in the healing callus when the ICP increased (p= 0.183). High ICP decreased the bone mineral density of the femoral head (p=0.005). CONCLUSION: High concentrations of pamidronate in intact bone decreased the bone mineral density and weakened the mechanical strength of the rat femora. The mechanical strength of the early healing callus was not correlated with concentration of pamidronate in the bone.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Anti-osteoporotic Drugs and Fracture Healing Mechanism
Kyu Hyun Yang
J Korean Fract Soc. 2011;24(2):212-216. doi: 10.12671/jkfs.2011.24.2.212.Difference in Bone Mineral Density Change at the Lateral Femoral Cortices according to Administration of Different Bisphosphonate Agents
Sungjun Kim, Hyun Hee Bang, Hanna Yoo, Il Hyung Park, Kyu Hyun Yang, Hyunsun Lim, Woo Seok Jung
J Bone Metab. 2016;23(2):85-93. doi: 10.11005/jbm.2016.23.2.85.
Reference
-
1. Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, et al. Ten year's experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004. 350:1189–1199.
Article2. Fleisch H. Bisphosphonates in bone disease. 2000. 4th ed. San Diego: Academic Press.3. Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005. 90:1294–1301.
Article4. Ott SM. Long-term safety of bisphosphonates. J Clin Endocrinol Metab. 2005. 90:1897–1899.
Article5. Maerevoet M, Martin C, Duck L. Osteonecrosis of the jaw and bisphosphonates. N Engl J Med. 2005. 353:99–102.
Article6. Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005. 63:1567–1575.
Article7. Komatsubara S, Mori S, Mashiba T, Ito M, Li J, Kaji Y, et al. Long-term treatment of incadronate disodium accumulates microdamage but improves the trabecular bone microarchitecture in dog vertebra. J Bone Miner Res. 2003. 18:512–520.
Article8. Whyte MP, Wenkert D, Clements KL, McAlister WH, Mumm S. Bisphosphonate-induced osteopetrosis. N Engl J Med. 2003. 349:457–463.
Article9. Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984. 2:97–101.
Article10. Hoggarth CR, Bennett R, Daley-Yates PT. The pharmacokinetics and distribution of pamidronate for a range of doses in the mouse. Calcif Tissue Int. 1991. 49:416–420.
Article11. Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993. 14:595–608.
Article12. King LE, Vieth R. Extraction and measurement of pamidronate from bone samples using automated pre-column derivatization, high-performance liquid chromatography and fluorescence detection. J Chromatogr B Biomed Appl. 1996. 678:325–330.
Article13. Cao Y, Mori S, Mashiba T, Westmore MS, Ma L, Sato M, et al. Raloxifene, estrogen, and alendronate affect the processes of fracture repair differently in ovariectomized rats. J Bone Miner Res. 2002. 17:2237–2246.
Article14. Goodship AE, Walker PC, Mc Nally D, Chambers T, Green JR. Use of a bisphosphonate (pamidronate) to modulate fracture repair in ovine bone. Ann Oncol. 1994. 5:Suppl 7. S53–S55.15. Koivukangas A, Tuukkanen J, Kippo K, Jämsä T, Hannuniemi R, Pasanen I, et al. Long-term administration of clodronate does not prevent fracture healing in rats. Clin Orthop Relat Res. 2003. 408:268–278.
Article16. Li J, Mori S, Kaji Y, Mashiba T, Kawanishi J, Norimatsu H. Effect of bisphosphonate (incadronate) on fracture healing of long bones in rats. J Bone Miner Res. 1999. 14:969–979.
Article17. Yang KH, Park SY, Yoo JH, Kim TH, Park HW, Ryu JH, et al. Effect of the long-term administration of pamidronate on bone strength and fracture healing in a rat model. J Korean Orthop Assoc. 2005. 40:1043–1049.18. Lehman RA, Kuklo TR, Freedman BA, Cowart JR, Mense MG, Riew KD. The effect of alendronate sodium on spinal fusion: a rabbit model. Spine J. 2004. 4:36–43.
Article19. Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998. 339:947–952.
Article20. Munns CF, Rauch F, Zeitlin L, Fassier F, Glorieux FH. Delayed osteotomy but not fracture healing in pediatric osteogenesis imperfecta patients receiving pamidronate. J Bone Miner Res. 2004. 19:1779–1786.
Article21. Liens D, Delmas PD, Meunier PJ. Long-term effects of intravenous pamidronate in fibrous dysplasia of bone. Lancet. 1994. 343:953–954.
Article22. Adamson BB, Gallacher SJ, Byars J, Ralston SH, Boyle IT, Boyce BF. Mineralisation defects with pamidronate therapy for Paget's disease. Lancet. 1993. 342:1459–1460.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Three - Dimensional Numerical analysis of the Mechanical function of the Plate Fixation for the Long bone Fracture
- The Effect of Disodium Pamidronate on Human Osteosarcoma Cells
- Mechanical and Morphological Properties of the Growing Long Bone: A Torsion Study on Rabbit's Femur
- Effects of whole-body vibration on bone properties in type 2 diabetes model rats
- Changes in the Microstructural and Mechanical Properties in the Medial Condyle of Human Distal Femur in Advanced Osteoarthritis