J Korean Med Sci.
2011 Mar;26(3):404-411. 10.3346/jkms.2011.26.3.404.
Pregabalin as a Neuroprotector after Spinal Cord Injury in Rats: Biochemical Analysis and Effect on Glial Cells
- Affiliations
-
- 1Department of Orthopaedic Surgery, College of Medicine, The Catholic University of Korea, Seoul, Korea. boscoa@empal.com
- 2Department of Orthopaedic Surgery, Stanford University, Stanford, CA, USA.
- KMID: 2157879
- DOI: http://doi.org/10.3346/jkms.2011.26.3.404
Abstract
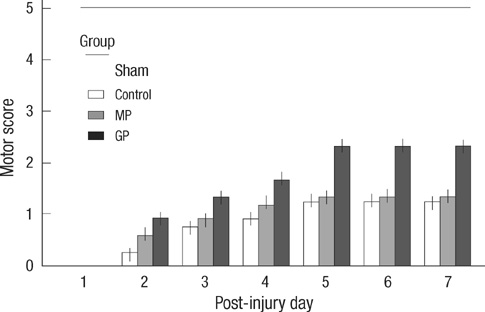
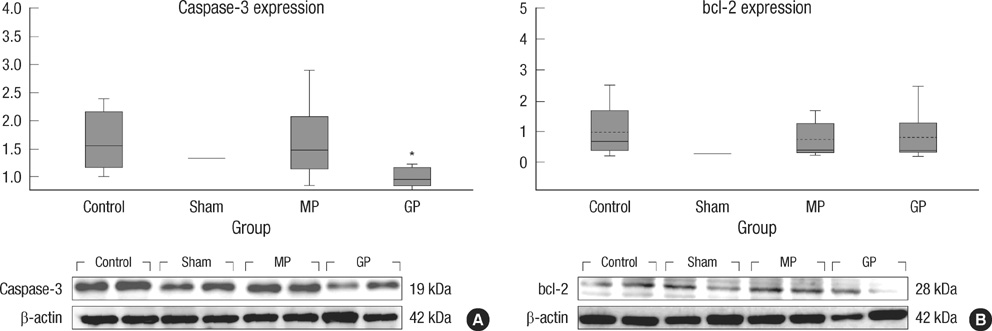
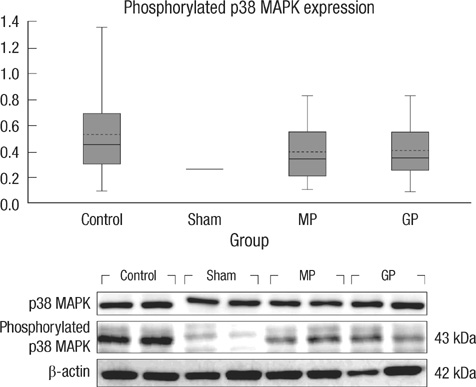
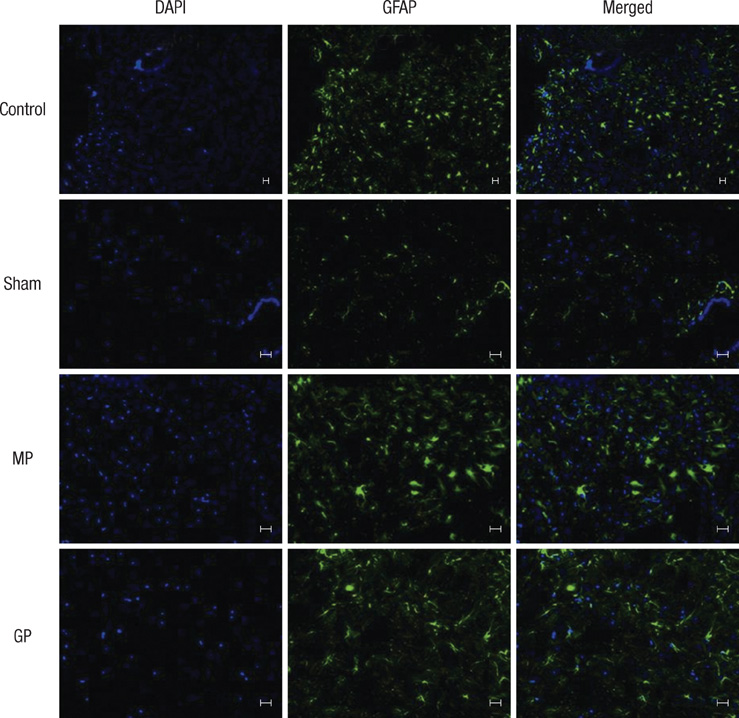
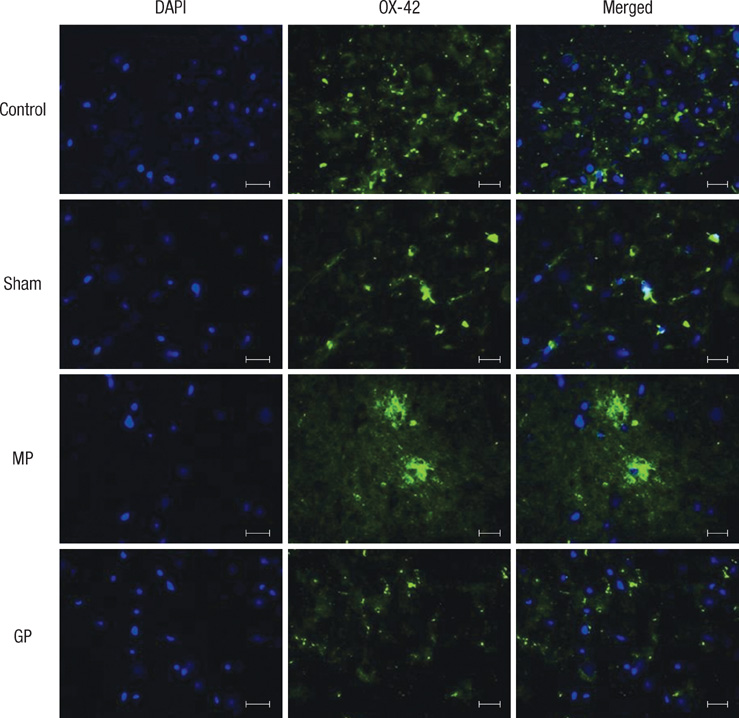
- As one of trials on neuroprotection after spinal cord injury, we used pregabalin. After spinal cord injury (SCI) in rats using contusion model, we observed the effect of pregabalin compared to that of the control and the methylprednisolone treated rats. We observed locomotor improvement of paralyzed hindlimb and body weight changes for clinical evaluation and caspase-3, bcl-2, and p38 MAPK expressions using western blotting. On histopathological analysis, we also evaluated reactive proliferation of glial cells. We were able to observe pregabalin's effectiveness as a neuroprotector after SCI in terms of the clinical indicators and the laboratory findings. The caspase-3 and phosphorylated p38 MAPK expressions of the pregabalin group were lower than those of the control group (statistically significant with caspase-3). Bcl-2 showed no significant difference between the control group and the treated groups. On the histopathological analysis, pregabalin treatment demonstrated less proliferation of the microglia and astrocytes. With this animal study, we were able to demonstrate reproducible results of pregabalin's neuroprotection effect. Diminished production of caspase-3 and phosphorylated p38 MAPK and as well as decreased proliferation of astrocytes were seen with the administration of pregabalin. This influence on spinal cord injury might be a possible approach for achieving neuroprotection following central nervous system trauma including spinal cord injury.
Keyword
MeSH Terms
-
Animals
Apoptosis/drug effects
Astrocytes/drug effects/pathology
Blotting, Western
Body Weight/drug effects
Caspase 3/genetics
Cell Proliferation
Fluorescent Antibody Technique
Gene Expression
Hindlimb/drug effects/pathology/physiopathology
Inflammation
Male
Methylprednisolone/therapeutic use
Microglia/drug effects/pathology
Motor Activity/drug effects
Neuroglia/*drug effects/pathology
Neuroprotective Agents/*therapeutic use
Paralysis/drug therapy
Proto-Oncogene Proteins c-bcl-2/genetics
Rats
Rats, Sprague-Dawley
Spinal Cord Injuries/*drug therapy/pathology
gamma-Aminobutyric Acid/*analogs & derivatives/therapeutic use
p38 Mitogen-Activated Protein Kinases/genetics
Figure
Cited by 2 articles
-
Spinal Cord Injury and Related Clinical Trials
Young-Hoon Kim, Kee-Yong Ha, Sang-Il Kim
Clin Orthop Surg. 2017;9(1):1-9. doi: 10.4055/cios.2017.9.1.1.Spinal Cord Injury and Neuro-Regeneration
Joo-Hyun Ahn, Hyung-Youl Park, Young-Hoon Kim
J Korean Orthop Assoc. 2019;54(6):498-508. doi: 10.4055/jkoa.2019.54.6.498.
Reference
-
1. Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004. 4:451–464.2. Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS. Acute spinal cord injury, Part I: pathophysiologic mechanisms. Clin Neuropharmacol. 2001. 24:254–264.3. Fehlings MG, Baptiste DC. Current status of clinical trials for acute spinal cord injury. Injury. 2005. 36:Suppl 2. B113–B122.4. Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008. 25:E2.5. Zhang Y, Bhavnani BR. Glutamate-induced apoptosis in primary cortical neurons is inhibited by equine estrogens via down-regulation of caspase-3 and prevention of mitochondrial cytochrome c release. BMC Neurosci. 2005. 6:13.6. Xu GY, Liu S, Hughes MG, McAdoo DJ. Glutamate-induced losses of oligodendrocytes and neurons and activation of caspase-3 in the rat spinal cord. Neuroscience. 2008. 153:1034–1047.7. Globus MY, Alonso O, Dietrich WD, Busto R, Ginsberg MD. Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia. J Neurochem. 1995. 65:1704–1711.8. Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004. 21:754–774.9. Káradóttir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005. 438:1162–1166.10. Joshi I, Taylor CP. Pregabalin action at a model synapse: binding to presynaptic calcium channel alpha2-delta subunit reduces neurotransmission in mice. Eur J Pharmacol. 2006. 553:82–88.11. Tassone DM, Boyce E, Guyer J, Nuzum D. Pregabalin: a novel gamma-aminobutyric acid analogue in the treatment of neuropathic pain, partial-onset seizures, and anxiety disorders. Clin Ther. 2007. 29:26–48.12. Fehrenbacher JC, Taylor CP, Vasko MR. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain. 2003. 105:133–141.13. Million M, Wang L, Adelson DW, Roman F, Diop L, Taché Y. Pregabalin decreases visceral pain and prevents spinal neuronal activation in rats. Gut. 2007. 56:1482–1484.14. Ha KY, Kim YH, Rhyu KW, Kwon SE. Pregabalin as a neuroprotector after spinal cord injury in rats. Eur Spine J. 2008. 17:864–872.15. Keane RW, Kraydieh S, Lotocki G, Bethea JR, Krajewski S, Reed JC, Dietrich WD. Apoptotic and anti-apoptotic mechanisms following spinal cord injury. J Neuropathol Exp Neurol. 2001. 60:422–429.16. Citron BA, Arnold PM, Haynes NG, Ameenuddin S, Farooque M, Santacruz K, Festoff BW. Neuroprotective effects of caspase-3 inhibition on functional recovery and tissue sparing after acute spinal cord injury. Spine (Phila Pa 1976). 2008. 33:2269–2277.17. Horiuchi H, Ogata T, Morino T, Chuai M, Yamamoto H. Continuous intrathecal infusion of SB203580, a selective inhibitor of p38 mitogen-activated protein kinase, reduces the damage of hind-limb function after thoracic spinal cord injury in rat. Neurosci Res. 2003. 47:209–217.18. Guo G, Bhat NR. p38alpha MAP kinase mediates hypoxia-induced motor neuron cell death: a potential target of minocycline's neuroprotective action. Neurochem Res. 2007. 32:2160–2166.19. Genovese T, Esposito E, Mazzon E, Muià C, Di Paola R, Meli R, Bramanti P, Cuzzocrea S. Evidence for the role of mitogen-activated protein kinase signaling pathways in the development of spinal cord injury. J Pharmacol Exp Ther. 2008. 325:100–114.20. Gale K, Kerasidis H, Wrathall JR. Spinal cord contusion in the rat; behavioral analysis of functional neurologic impairment. Exp Neurol. 1985. 88:123–134.21. Austin JW, Fehlings MG. Molecular mechanisms of Fas-mediated cell death in oligodendrocytes. J Neurotrauma. 2008. 25:411–426.22. Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, Fukuoka T, Tokunaga A, Noguchi K. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J Neurosci. 2004. 24:10211–10222.23. Nakahara S, Yone K, Sakou T, Wada S, Nagamine T, Niiyama T, Ichijo H. Induction of apoptosis signal regulating kinase 1 (ASK1) after spinal cord injury in rats: possible involvement of ASK1-JNK and -p38 pathways in neuronal apoptosis. J Neuropathol Exp Neurol. 1999. 58:442–450.24. Kwak EK, Kim JW, Kang KS, Lee YH, Hua QH, Park TI, Park JY, Sohn YK. The role of inducible nitric oxide synthase following spinal cord injury in rat. J Korean Med Sci. 2005. 20:663–669.25. Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000. 32:1–14.26. Williams A, Piaton G, Lubetzki C. Astrocytes--friends or foes in multiple sclerosis? Glia. 2007. 55:1300–1312.27. Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004. 24:2143–2155.28. Barritt AW, Davies M, Marchand F, Hartley R, Grist J, Yip P, McMahon SB, Bradbury EJ. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci. 2006. 26:10856–10867.29. Buffo A, Rolando C, Ceruti S. Astrocytes in the damaged brain: molecular and cellular insights into their reactive response and healing potential. Biochem Pharmacol. 2010. 79:77–89.30. Rosenstein JM, Krum JM. New roles for VEGF in nervous tissue-beyond blood vessels. Exp Neurol. 2004. 187:246–253.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Activation of Embryonic Intermediate Filaments Contributes to Glial Scar Formation after Spinal Cord Injury in Rats
- Effects of Fetal Spinal Cord Transplants on Injured Rat Spinal Cord
- Functional Recovery in Complete Spinal Cord Injury after Transplantation of Human Umbilical Cord Blood Cells in Rats
- Transplantation of Human Umbilical Cord Blood Improves Neurological Outcomes in the Rats after Traumatic Spinal Cord Injury
- Cell Death in Acute Spinal Cord Injury