J Vet Sci.
2014 Sep;15(3):399-407. 10.4142/jvs.2014.15.3.399.
Enhancing mucosal immunity in mice by recombinant adenovirus expressing major epitopes of porcine circovirus-2 capsid protein delivered with cytosine-phosphate-guanosine oligodeoxynucleotides
- Affiliations
-
- 1Animal Infectious Disease Lab, College of Animal Science and Veterinary Medicine, Henan Agricultural University, Zhengzhou 450002, China. chenluhau@hotmail.com
- KMID: 2155623
- DOI: http://doi.org/10.4142/jvs.2014.15.3.399
Abstract
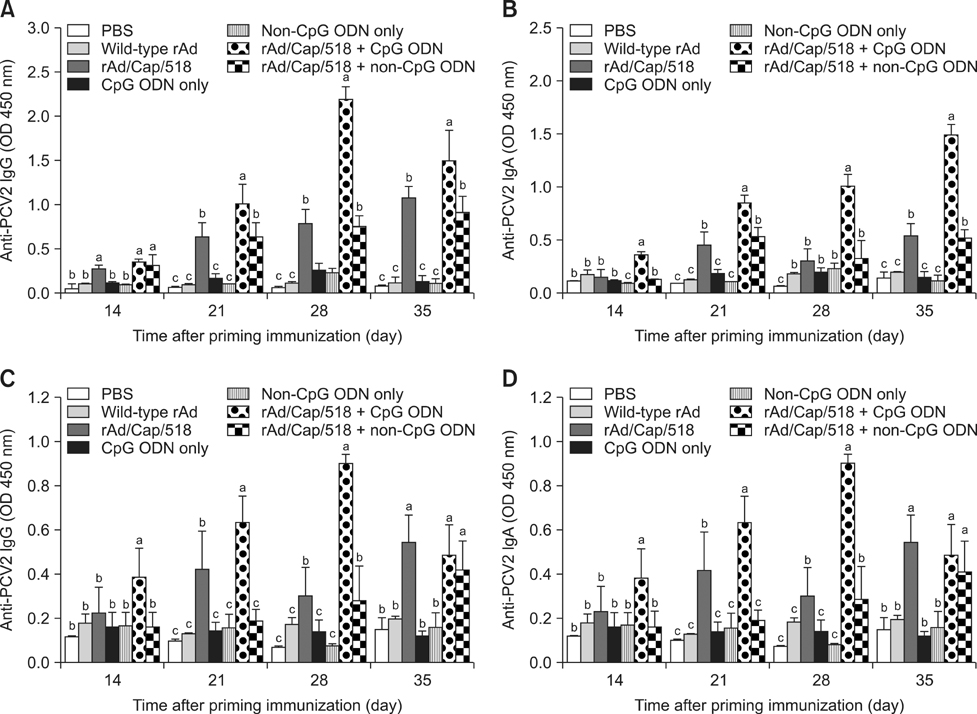
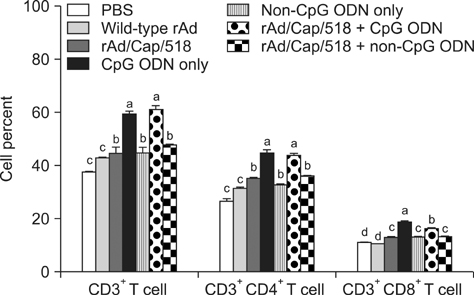
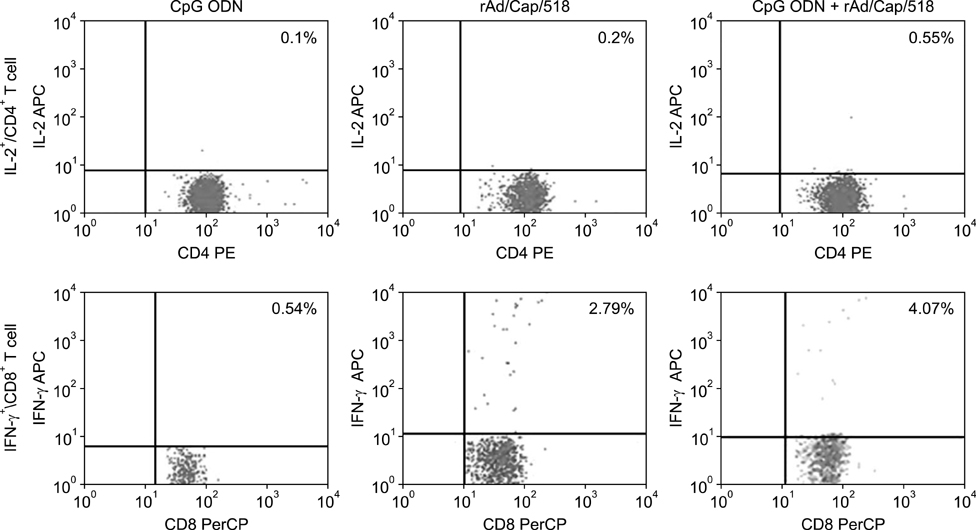
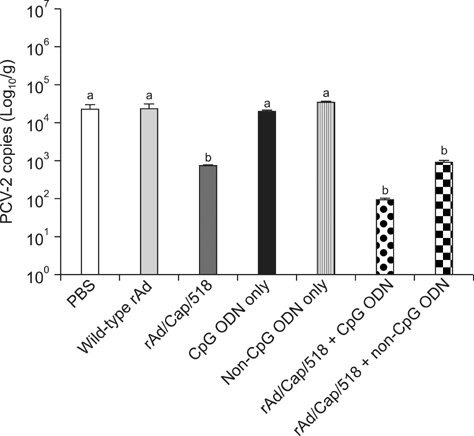
- A recombinant replication-defective adenovirus expressing the major epitopes of porcine circovirus-2 (PCV-2) capsid protein (rAd/Cap/518) was previously constructed and shown to induce mucosal immunity in mice following intranasal delivery. In the present study, immune responses induced by intranasal immunization with a combination of rAd/Cap/518 and cytosine-phosphate-guanosine oligodeoxynucleotides (CpG ODN) were evaluated in mice. The levels of PCV-2-specific IgG in serum and IgA in saliva, lung, and intestinal fluids were significantly higher in the group immunized with rAd/Cap/518 and CpG ODN than animals immunized with rAd/Cap/518 alone. The frequencies of IL-2-secreting CD4+ T cells and IFN-gamma-producing CD8+ T cells were significantly higher in the combined immunization group than mice immunized with rAd/Cap/518 alone. The frequencies of CD3+, CD3+CD4+CD8-, and CD3+CD4-CD8+ T cells in the combined immunization group were similar to that treated with CpG ODN alone, but significantly higher than mice that did not receive CpG ODN. PCV-2 load after challenge in the combined immunization group was significantly lower than that in the phosphate-buffered saline placebo group and approximately 7-fold lower in the group treated with CpG ODN alone. These results indicate that rAd/Cap/518 combined with CpG ODN can enhance systemic and local mucosal immunity in mice, and represent a promising synergetic mucosal vaccine against PCV-2.
Keyword
MeSH Terms
-
Adenoviridae/genetics/immunology
Administration, Intranasal
Animals
Capsid Proteins/*genetics/immunology
Circoviridae Infections/*immunology
Circovirus/*genetics/immunology
Epitopes/genetics/immunology
Female
Immunity, Mucosal/immunology
Immunoglobulin A/blood/immunology
Immunoglobulin G/blood/immunology
Mice
Mice, Inbred BALB C
Oligodeoxyribonucleotides/genetics
Vaccines, Synthetic/genetics/immunology
Viral Vaccines/administration & dosage/*genetics/immunology
Capsid Proteins
Epitopes
Immunoglobulin A
Immunoglobulin G
Oligodeoxyribonucleotides
Vaccines, Synthetic
Viral Vaccines
Figure
Reference
-
1. Alarcon P, Rushton J, Wieland B. Cost of post-weaning multi-systemic wasting syndrome and porcine circovirus type-2 subclinical infection in England - an economic disease model. Prev Vet Med. 2013; 110:88–102.
Article2. Appledorn DM, Aldhamen YA, Godbehere S, Seregin SS, Amalfitano A. Sublingual administration of an adenovirus serotype 5 (Ad5)-based vaccine confirms Toll-like receptor agonist activity in the oral cavity and elicits improved mucosal and systemic cell-mediated responses against HIV antigens despite preexisting Ad5 immunity. Clin Vaccine Immunol. 2011; 18:150–160.
Article3. Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011; 10:499–511.
Article4. Cheung AK. Transcriptional analysis of porcine circovirus type 2. Virology. 2003; 305:168–180.
Article5. Croyle MA, Patel A, Tran KN, Gray M, Zhang Y, Strong JE, Feldmann H, Kobinger GP. Nasal delivery of an adenovirus-based vaccine bypasses pre-existing immunity to the vaccine carrier and improves the immune response in mice. PLoS One. 2008; 3:e3548.
Article6. Cságola A, Cadar D, Tuboly T. Replication and transmission of porcine circovirus type 2 in mice. Acta Vet Hung. 2008; 56:421–427.
Article7. Deng ZB, Wang ND, Xu DJ, Yuan AW, Ge M, Luo W, Xue LQ, Yu XL. Viral distribution and lesions in Kunming mice experimentally infected with porcine circovirus type 2b. Vet Res Commun. 2011; 35:181–192.
Article8. Ellis J, Clark E, Haines D, West K, Krakowka S, Kennedy S, Allan GM. Porcine circovirus-2 and concurrent infections in the field. Vet Microbiol. 2004; 98:159–163.
Article9. Fujkuyama Y, Tokuhara D, Kataoka K, Gilbert RS, McGhee JR, Yuki Y, Kiyono H, Fujihashi K. Novel vaccine development strategies for inducing mucosal immunity. Expert Rev Vaccines. 2012; 11:367–379.
Article10. Gallichan WS, Woolstencroft RN, Guarasci T, McCluskie MJ, Davis HL, Rosenthal KL. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J Immunol. 2001; 166:3451–3457.
Article11. Guo QH, Chen L, Wang CQ, Wu DF, Wang YS, Li ZJ, Wang Y. Construction and identification of recombinant adenoviruses containing the ORF2 gene of porcine circovirus 2. Chin J Vet Sci. 2011; 31:1099–1102. 111012. Grau-Roma L, Hjulsager CK, Sibila M, Kristensen CS, López-Soria S, Enøe C, Casal J, Bøtner A, Nofrarías M, Bille-Hansen V, Fraile L, Baekbo P, Segalés J, Larsen LE. Infection, excretion and seroconversion dynamics of porcine circovirus type 2 (PCV2) in pigs from post-weaning multisystemic wasting syndrome (PMWS) affected farms in Spain and Denmark. Vet Microbiol. 2009; 135:272–282.
Article13. Hanagata N. Structure-dependent immunostimulatory effect of CpG oligodeoxynucleotides and their delivery system. Int J Nanomedicine. 2012; 7:2181–2195.
Article14. Harandi AM, Eriksson K, Holmgren J. A protective role of locally administered immunostimulatory CpG oligodeoxynucleotide in a mouse model of genital herpes infection. J Virol. 2003; 77:953–962.
Article15. He J, Cao J, Zhou N, Jin Y, Wu J, Zhou J. Identification and functional analysis of the novel ORF4 protein encoded by porcine circovirus type 2. J Virol. 2013; 87:1420–1429.
Article16. Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000; 408:740–745.
Article17. Kim S, Jang JE, Yu JR, Chang J. Single mucosal immunization of recombinant adenovirus-based vaccine expressing F1 protein fragment induces protective mucosal immunity against respiratory syncytial virus infection. Vaccine. 2010; 28:3801–3808.
Article18. Kiupel M, Stevenson GW, Choi J, Latimer KS, Kanitz CL, Mittal SK. Viral replication and lesions in BALB/c mice experimentally inoculated with porcine circovirus isolated from a pig with postweaning multisystemic wasting disease. Vet Pathol. 2001; 38:74–82.
Article19. Kiupel M, Stevenson GW, Galbreath EJ, North A, HogenEsch H, Mittal SK. Porcine circovirus type 2 (PCV2) causes apoptosis in experimentally inoculated BALB/c mice. BMC Vet Res. 2005; 1:7.
Article20. Klinman DM, Klaschik S, Sato T, Tross D. CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases. Adv Drug Deliv Rev. 2009; 61:248–255.
Article21. Ko SY, Cheng C, Kong WP, Wang L, Kanekiyo M, Einfeld D, King CR, Gall JG, Nabel GJ. Enhanced induction of intestinal cellular immunity by oral priming with enteric adenovirus 41 vectors. J Virol. 2009; 83:748–756.
Article22. Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995; 374:546–549.
Article23. Krishnamachari Y, Salem AK. Innovative strategies for co-delivering antigens and CpG oligonucleotides. Adv Drug Deliv Rev. 2009; 61:205–217.
Article24. Lekcharoensuk P, Morozov I, Paul PS, Thangthumniyom N, Wajjawalku W, Meng XJ. Epitope mapping of the major capsid protein of type 2 porcine circovirus (PCV2) by using chimeric PCV1 and PCV2. J Virol. 2004; 78:8135–8145.
Article25. Li Z, Wang Y, Chen L, Yang X, Zhao J, Wang C. Study and application of SYBR green I-based fluorescent quantitative PCR assay in type 2 porcine circovirus detection. Yang zhou da xue xue bao. Nong ye yu sheng ming ke xue ban. 2011; 32:49–54.26. Lin SW, Cun AS, Harris-McCoy K, Ertl HC. Intramuscular rather than oral administration of replication-defective adenoviral vaccine vector induces specific CD8+ T cell responses in the gut. Vaccine. 2007; 25:2187–2193.
Article27. Liu J, Chen I, Du Q, Chua H, Kwang J. The ORF3 protein of porcine circovirus type 2 is involved in viral pathogenesis in vivo. J Virol. 2006; 80:5065–5073.
Article28. Liu YF, Guo QH, Chen L, Zhao J, Chang HT, Wang XW, Yang X, Wang CQ. Induction of mucosal immunity by intranasal immunization with recombinant adenovirus expressing major epitopes of Porcine circovirus-2 capsid protein. Vet Immunol Immunopathol. 2013; 154:48–53.
Article29. Liu Y, Ma P, Chen L, Yang X, Yao H, Wang C, Zhao J. Development and primary application of indirect ELISA method for detecting the IgA against porcine circovirus type 2. Chin J Prev Vet Med. 2012; 34:301–304.30. Loeffen W. Persistent infection with the classical swine fever virus in vaccinated animals: a risk factor? Tijdschr Diergeneeskd. 2008; 133:482–484.31. Naeem K, Naurin M, Rashid S, Bano S. Seroprevalence of avian influenza virus and its relationship with increased mortality and decreased egg production. Avian Pathol. 2003; 32:285–289.
Article32. Nawagitgul P, Morozov I, Bolin SR, Harms PA, Sorden SD, Paul PS. Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J Gen Virol. 2000; 81:2281–2287.
Article33. Patterson AR, Madson DM, Halbur PG, Opriessnig T. Shedding and infection dynamics of porcine circovirus type 2 (PCV2) after natural exposure. Vet Microbiol. 2011; 149:225–229.
Article34. Pavot V, Rochereau N, Genin C, Verrier B, Paul S. New insights in mucosal vaccine development. Vaccine. 2012; 30:142–154.
Article35. Takada A, Kida H. Protective immune response of chickens against Newcastle disease, induced by the intranasal vaccination with inactivated virus. Vet Microbiol. 1996; 50:17–25.
Article36. Tutykhina IL, Logunov DY, Shcherbinin DN, Shmarov MM, Tukhvatulin AI, Naroditsky BS, Gintsburg AL. Development of adenoviral vector-based mucosal vaccine against influenza. J Mol Med (Berl). 2011; 89:331–341.
Article37. Woodrow KA, Bennett KM, Lo DD. Mucosal vaccine design and delivery. Annu Rev Biomed Eng. 2012; 14:17–46.
Article38. Xiang ZQ, Gao GP, Reyes-Sandoval A, Li Y, Wilson JM, Ertl HC. Oral vaccination of mice with adenoviral vectors is not impaired by preexisting immunity to the vaccine carrier. J Virol. 2003; 77:10780–10789.
Article39. Yoon HA, Aleyas AG, George JA, Park SO, Han YW, Hyun BH, Lee JH, Song HJ, Cho JG, Eo SK. Correlation between the nature of immunity induced by different immunogens and the establishment of latent infection by wild-type pseudorabies virus. Res Vet Sci. 2007; 83:73–81.
Article40. Zhao J, Lai L, Amara RR, Montefiori DC, Villinger F, Chennareddi L, Wyatt LS, Moss B, Robinson HL. Preclinical studies of human immunodeficiency virus/AIDS vaccines: inverse correlation between avidity of anti-Env antibodies and peak postchallenge viremia. J Virol. 2009; 83:4102–4111.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Enhanced protective immune response to PCV2 adenovirus vaccine by fusion expression of Cap protein with InvC in pigs
- Immunogenicity of a Canine Parvovirus 2 Capsid Antigen (VP2-S1) surface-expressed on Lactobacillus casei
- Expression and evaluation of porcine circovirus type 2 capsid protein mediated by recombinant adenoassociated virus 8
- Genetic variations in open reading frame 2 gene of porcine circovirus type 2 isolated in Korea during 2016–2017
- Construction and Characterization of Recombinant Poliovirus that Delivers T-cell epitope