J Vet Sci.
2014 Sep;15(3):335-342. 10.4142/jvs.2014.15.3.335.
Comparison of arylalkylamine N-acetyltransferase and melatonin receptor type 1B immunoreactivity between young adult and aged canine spinal cord
- Affiliations
-
- 1Department of Neurobiology, School of Medicine, Kangwon National University, Chuncheon 200-701, Korea. hcshin@hallym.ac.kr
- 2Institute of Integrative Traditional and Western Medicine, Medical College, Yangzhou University, Yangzhou 225001, China.
- 3Laboratory of Neuroscience, Department of Physical Therapy, College of Rehabilitation Science, Daegu University, Gyeongsan 712-714, Korea.
- 4Department of Pharmacy, College of Pharmacy, Dankook University, Cheonan 330-714, Korea.
- 5Department of Anatomy, College of Veterinary Medicine, Kangwon National University, Chuncheon 200-701, Korea.
- 6Department of Oral Anatomy, College of Dentistry, Gangneung-Wonju National University, Gangneung 210-702, Korea.
- 7Department of Anatomy and Cell Biology, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea.
- 8Department of Neurosurgery, Dongtan Sacred Heart Hospital, College of Medicine, Hallym University, Hwaseong 445-170, Korea.
- 9Department of Physiology, College of Medicine, Hallym University, Chuncheon 200-702, Korea. hcshin@hallym.ac.kr
- KMID: 2155616
- DOI: http://doi.org/10.4142/jvs.2014.15.3.335
Abstract
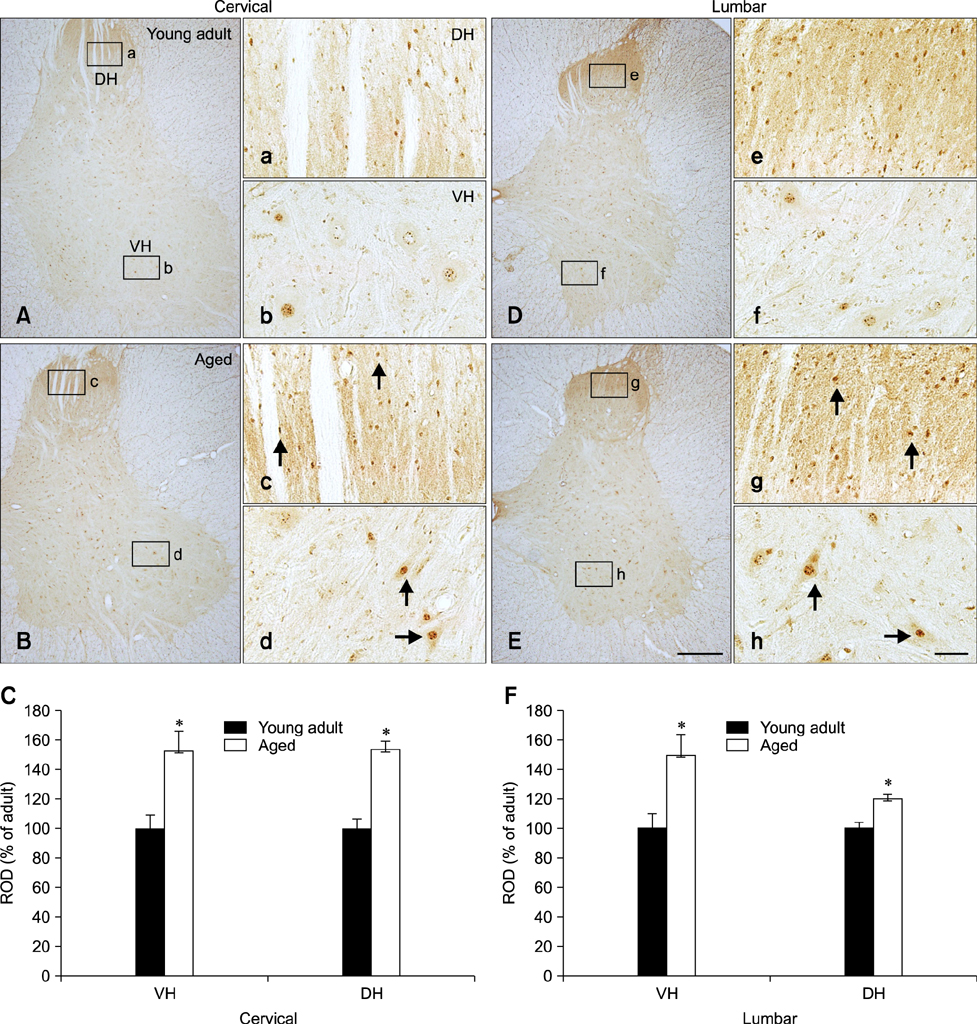
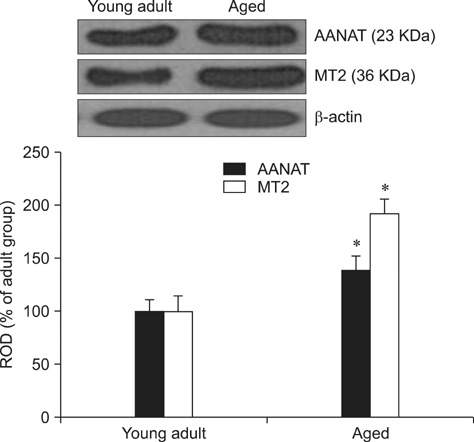
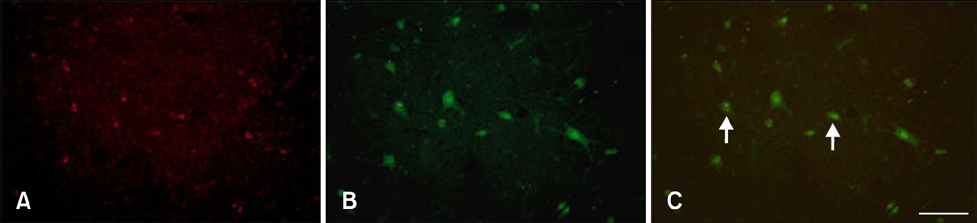
- Melatonin affects diverse physiological functions through its receptor and plays an important role in the central nervous system. In the present study, we compared immunoreactivity patterns of arylalkylamine N-acetyltransferase (AANAT), an enzyme essential for melatonin synthesis, and melatonin receptor type 1B (MT2) in the spinal cord of young adult (2~3 years) and aged (10~12 years) beagle dogs using immunohistochemistry and Western blotting. AANAT-specific immunoreactivity was observed in the nuclei of spinal neurons, and was significantly increased in aged dog spinal neurons compared to young adult spinal neurons. MT2-specific immunoreactivity was found in the cytoplasm of spinal neurons, and was predominantly increased in the margin of the neuron cytoplasm in aged spinal cord compared to that in the young adult dogs. These increased levels of AANAT and MT2 immunoreactivity in aged spinal cord might be a feature of normal aging and associated with a feedback mechanism that compensates for decreased production of melatonin during aging.
MeSH Terms
-
Age Factors
Aging/physiology
Animals
Arylalkylamine N-Acetyltransferase/*analysis/immunology/physiology
Blotting, Western
Dogs
Fluorescent Antibody Technique
Male
Receptor, Melatonin, MT2/*analysis/immunology/physiology
Spinal Cord/*chemistry/immunology/physiology
Arylalkylamine N-Acetyltransferase
Receptor, Melatonin, MT2
Figure
Reference
-
1. Ahn JH, Choi JH, Song JM, Lee CH, Yoo KY, Hwang IK, Kim JS, Shin HC, Won MH. Increase in Trx2/Prx3 redox system immunoreactivity in the spinal cord and hippocampus of aged dogs. Exp Gerontol. 2011; 46:946–952.
Article2. Cotman CW, Head E. The canine (dog) model of human aging and disease: dietary, environmental and immunotherapy approaches. J Alzheimers Dis. 2008; 15:685–707.
Article3. Dagci T, Yilmaz O, Taskiran D, Peker G. Neuroprotective agents: is effective on toxicity in glial cells? Cell Mol Neurobiol. 2007; 27:171–177.
Article4. Dominguez-Rodriguez A, Abreu-Gonzalez P, Sanchez-Sanchez JJ, Kaski JC. Melatonin and circadian biology in human cardiovascular disease. J Pineal Res. 2010; 49:14–22.
Article5. Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005; 27:101–110.6. Espino J, Pariente JA, Rodríguez AB. Oxidative stress and immunosenescence: therapeutic effects of melatonin. Oxid Med Cell Longev. 2012; 2012:670294.
Article7. Esposito E, Cuzzocrea S. Antiinflammatory activity of melatonin in central nervous system. Curr Neuropharmacol. 2010; 8:228–242.
Article8. Ganguly S, Coon SL, Klein DC. Control of melatonin synthesis in the mammalian pineal gland: the critical role of serotonin acetylation. Cell Tissue Res. 2002; 309:127–137.
Article9. Guerrero HY, Gauer F, Schuster C, Pévet P, Masson-Pévet M. Melatonin regulates the mRNA expression of the mt1 melatonin receptor in the rat Pars tuberalis. Neuroendocrinology. 2000; 71:163–169.
Article10. Hardeland R. Melatonin in aging and disease-multiple consequences of reduced secretion, options and limits of treatment. Aging Dis. 2012; 3:194–225.11. Jagota A, Reddy MY. The effect of curcumin on ethanol induced changes in suprachiasmatic nucleus (SCN) and pineal. Cell Mol Neurobiol. 2007; 27:997–1006.
Article12. Karasek M. Melatonin, human aging, and age-related diseases. Exp Gerontol. 2004; 39:1723–1729.
Article13. Lee CH, Choi JH, Yoo KY, Park OK, Hwang IK, You SG, Lee BY, Kang IJ, Won MH. MT2 melatonin receptor immunoreactivity in neurons is very high in the aged hippocampal formation in gerbils. Cell Mol Neurobiol. 2010; 30:255–263.
Article14. Lee DH, Ahn JH, Park JH, Yan BC, Cho JH, Kim IH, Lee JC, Jang SH, Lee MH, Hwang IK, Moon SM, Lee B, Cho JH, Shin HC, Kim JS, Won MH. Comparison of expression of inflammatory cytokines in the spinal cord between young adult and aged beagle dogs. Cell Mol Neurobiol. 2013; 33:615–624.
Article15. Maronde E, Saade A, Ackermann K, Goubran-Botros H, Pagan C, Bux R, Bourgeron T, Dehghani F, Stehle JH. Dynamics in enzymatic protein complexes offer a novel principle for the regulation of melatonin synthesis in the human pineal gland. J Pineal Res. 2011; 51:145–155.
Article16. National Research Council of the National Academies (US). Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, D.C: National Academies Press;2011.17. Piesiewicz A, Kedzierska U, Podobas E, Adamska I, Zuzewicz K, Majewski PM. Season-dependent postembryonic maturation of the diurnal rhythm of melatonin biosynthesis in the chicken pineal gland. Chronobiol Int. 2012; 29:1227–1238.
Article18. Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev. 1991; 12:151–180.
Article19. Rodrigues de Amorim MA, Garcia-Segura LM, Goya RG, Portiansky EL. Decrease in PTEN and increase in Akt expression and neuron size in aged rat spinal cord. Exp Gerontol. 2010; 45:457–463.
Article20. Sarasa M, Pesini P. Natural non-trasgenic animal models for research in Alzheimer's disease. Curr Alzheimer Res. 2009; 6:171–178.
Article21. Schuster C, Gauer F, Malan A, Recio J, Pévet P, Masson-Pévet M. The circadian clock, light/dark cycle and melatonin are differentially involved in the expression of daily and photoperiodic variations in mt1 melatonin receptors in the Siberian and Syrian hamsters. Neuroendocrinology. 2001; 74:55–68.
Article22. Stefulj J, Hörtner M, Ghosh M, Schauenstein K, Rinner I, Wölfler A, Semmler J, Liebmann PM. Gene expression of the key enzymes of melatonin synthesis in extrapineal tissues of the rat. J Pineal Res. 2001; 30:243–247.
Article23. Uz T, Qu T, Sugaya K, Manev H. Neuronal expression of arylalkylamine N-acetyltransferase (AANAT) mRNA in the rat brain. Neurosci Res. 2002; 42:309–316.
Article24. Wan Q, Liao M, Brown GM, Pang SF. Localization and characterization of melatonin receptors in the rabbit spinal cord. Neurosci Lett. 1996; 204:77–80.
Article25. Wan Q, Pang SF. Segmental, coronal and subcellular distribution of 2-[125I]iodomelatonin binding sites in the chicken spinal cord. Neurosci Lett. 1994; 180:253–256.
Article26. Wilhelmsen M, Amirian I, Reiter RJ, Rosenberg J, Gögenur I. Analgesic effects of melatonin: a review of current evidence from experimental and clinical studies. J Pineal Res. 2011; 51:270–277.
Article27. Wu J, Hu Q, Huang D, Chen X, Chen J. Effect of electrical stimulation of sciatic nerve on synaptic plasticity of spinal dorsal horn and spinal c-fos expression in neonatal, juvenile and adult rats. Brain Res. 2012; 1448:11–19.
Article28. Yu CX, Zhu CB, Xu SF, Cao XD, Wu GC. Selective MT2 melatonin receptor antagonist blocks melatonin-induced antinociception in rats. Neurosci Lett. 2000; 282:161–164.
Article29. Yuan Q, Su H, Guo J, Tsang KY, Cheah KS, Chiu K, Yang J, Wong WM, So KF, Huang JD, Wu W, Lin ZX. Decreased c-Jun expression correlates with impaired spinal motoneuron regeneration in aged mice following sciatic nerve crush. Exp Gerontol. 2012; 47:329–336.
Article30. Zahn PK, Lansmann T, Berger E, Speckmann EJ, Musshoff U. Gene expression and functional characterization of melatonin receptors in the spinal cord of the rat: implications for pain modulation. J Pineal Res. 2003; 35:24–31.
Article31. Zheng W, Cole PA. Serotonin N-acetyltransferase: mechanism and inhibition. Curr Med Chem. 2002; 9:1187–1199.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of alpha-synuclein immunoreactivity in the spinal cord between the adult and aged beagle dog
- Melatonin ameliorates autoimmune encephalomyelitis through suppression of intercellular adhesion molecule-1
- Immunoreactivity of Androgen Receptor Protein in Sexually Dimorphic Spinal Motonucleus in Neonatal Male Rats
- Spatial and temporal expression patterns of apoptosis-related genes in rat spinal cord during normal aging
- Immunoreactivity of androgen receptor protein in sexually dimorphic spinal motonucleus in neonatal male rats