Korean J Radiol.
2015 Jun;16(3):575-585. 10.3348/kjr.2015.16.3.575.
Evaluation of Engraftment of Superparamagnetic Iron Oxide-Labeled Mesenchymal Stem Cells Using Three-Dimensional Reconstruction of Magnetic Resonance Imaging in Photothrombotic Cerebral Infarction Models of Rats
- Affiliations
-
- 1Department of Convergence Medicine and Pharmaceutical Biosciences, Chung-Ang University, Seoul 156-756, Korea.
- 2Department of Radiology, Chung-Ang University Hospital, Chung-Ang University College of Medicine, Seoul 156-755, Korea. kwakbk@cau.ac.kr
- 3Major of Biomedical Science, Chung-Ang University College of Medicine, Seoul 156-756, Korea.
- KMID: 2155527
- DOI: http://doi.org/10.3348/kjr.2015.16.3.575
Abstract
OBJECTIVE
To evaluate engraftment by visualizing the location of human bone marrow-derived mesenchymal stem cells (hBM-MSCs) three-dimensionally in photothrombotic cerebral infarction (PTCI) models of rats.
MATERIALS AND METHODS
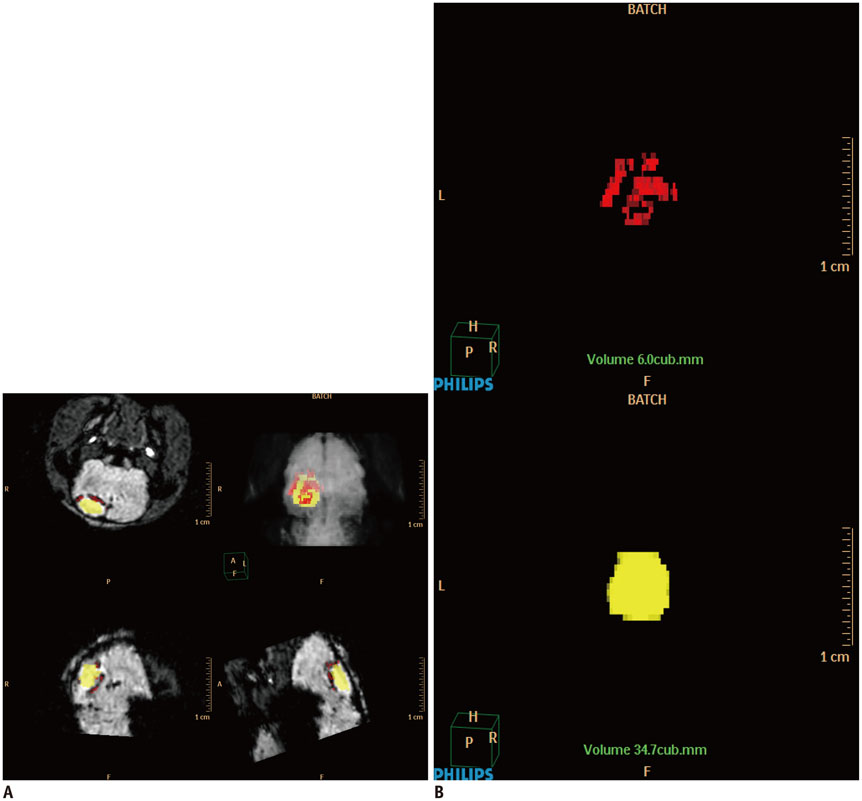
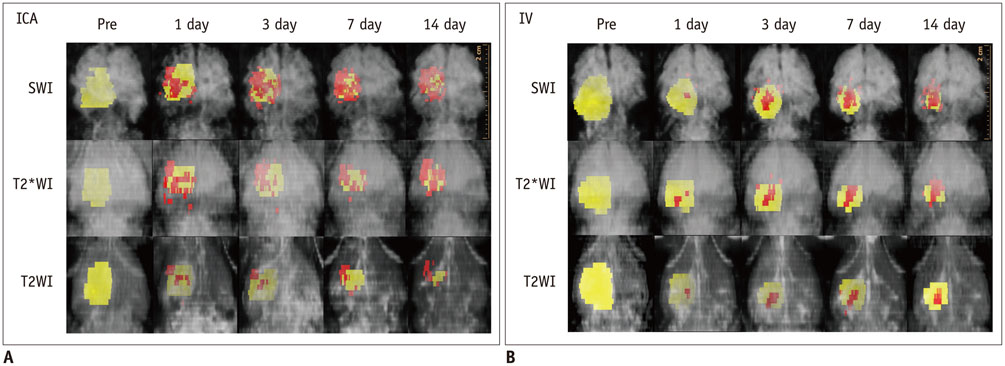
Magnetic resonance imaging (MRI) of an agarose block containing superparamagnetic iron oxide (SPIO)-labeled hBM-MSCs was performed using a 3.0-T MRI, T2-(T2WI), T2*-(T2*WI), and susceptibility-weighted images (SWI). PTCI was induced in 6 rats, and 2.5 x 10(5) SPIO-labeled hBM-MSCs were infused through the ipsilateral internal carotid artery (ICA group) or tail vein (IV group). MRI was performed on days 1, 3, 7, and 14 after stem cell injection. Dark signal regions were confirmed using histology. Three-dimensional MRI reconstruction was performed using the clinical workflow solution to evaluate the engraftment of hBM-MSCs. Volumetric analysis of the engraftment was also performed.
RESULTS
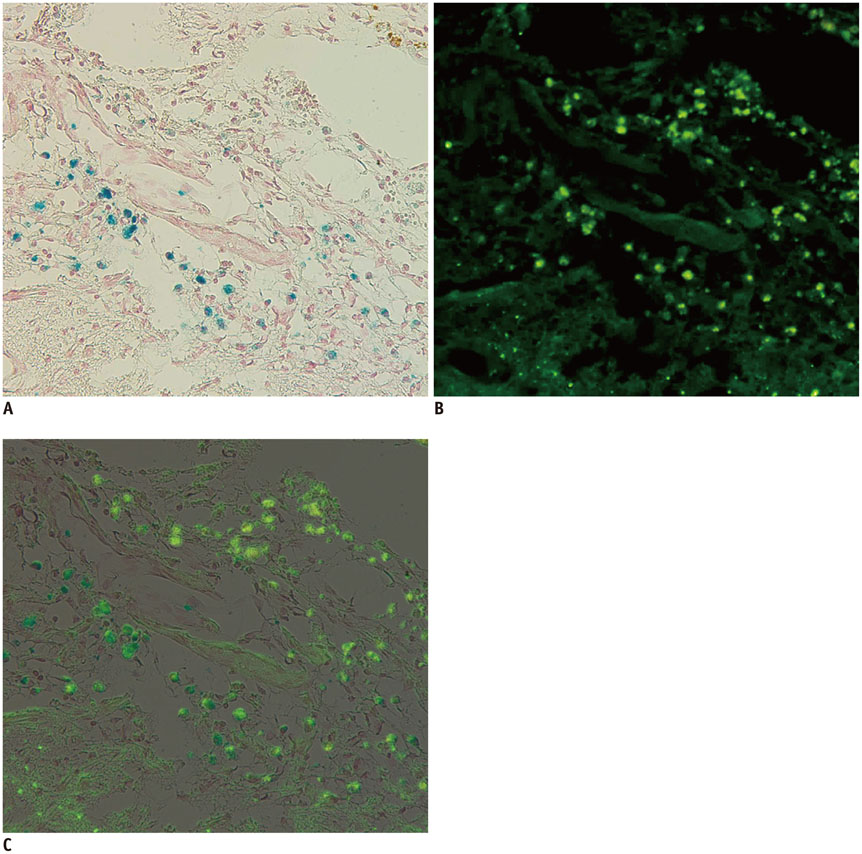
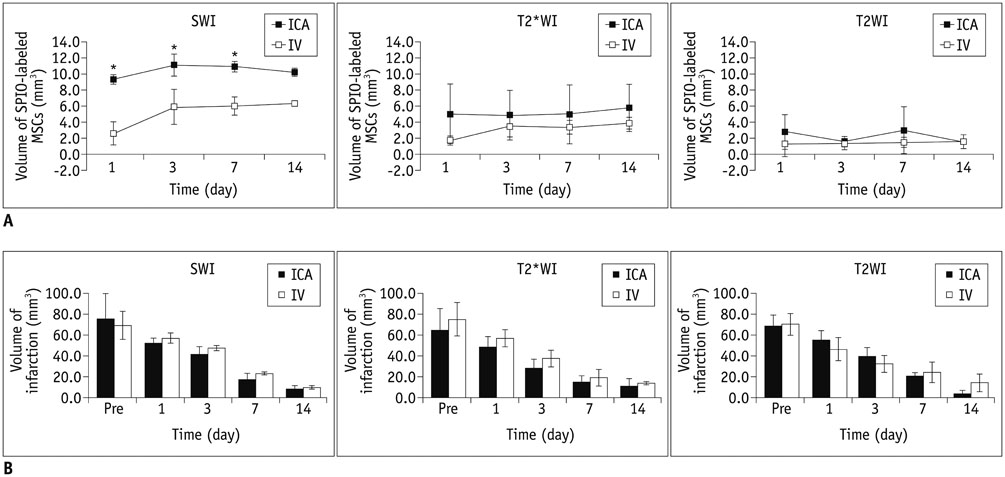
The volumes of SPIO-labeled hBM-MSCs in the phantom MRI were 129.3, 68.4, and 25.9 microL using SWI, T2*WI, and T2WI, respectively. SPIO-labeled hBM-MSCs appeared on day 1 after injection, encircling the cerebral infarction from the ventral side. Dark signal regions matched iron positive cells and human origin (positive) cells. The volume of the engraftment was larger in the ICA group on days 1, 3, and 7, after stem cell injection (p < 0.05 on SWI). SWI was the most sensitive MRI pulse sequence (p < 0.05). The volume of infarction decreased until day 14.
CONCLUSION
The engraftment of SPIO-labeled hBM-MSCs can be visualized and evaluated three-dimensionally in PTCI models of rats. The engraftment volume was larger in the ICA group than IV group on early stage within one week.
Keyword
MeSH Terms
-
Animals
Cerebral Infarction/pathology/*radiography
Contrast Media
Dextrans
Humans
Imaging, Three-Dimensional/methods
Magnetic Resonance Imaging/*methods
Magnetite Nanoparticles
Male
*Mesenchymal Stem Cell Transplantation
Mesenchymal Stromal Cells/radiography
Nanoparticles
Neuroimaging/*methods
Random Allocation
Rats
Rats, Sprague-Dawley
Tomography, X-Ray Computed
Contrast Media
Dextrans
Magnetite Nanoparticles
Figure
Reference
-
1. Yang Y, Zhang J, Qian Y, Dong S, Huang H, Boada FE, et al. Superparamagnetic iron oxide is suitable to label tendon stem cells and track them in vivo with MR imaging. Ann Biomed Eng. 2013; 41:2109–2119.2. Bulte JW. In vivo MRI cell tracking: clinical studies. AJR Am J Roentgenol. 2009; 193:314–325.3. Richards JM, Shaw CA, Lang NN, Williams MC, Semple SI, MacGillivray TJ, et al. In vivo mononuclear cell tracking using superparamagnetic particles of iron oxide: feasibility and safety in humans. Circ Cardiovasc Imaging. 2012; 5:509–517.4. Hu SL, Lu PG, Zhang LJ, Li F, Chen Z, Wu N, et al. In vivo magnetic resonance imaging tracking of SPIO-labeled human umbilical cord mesenchymal stem cells. J Cell Biochem. 2012; 113:1005–1012.5. Reddy AM, Kwak BK, Shim HJ, Ahn C, Cho SH, Kim BJ, et al. Functional characterization of mesenchymal stem cells labeled with a novel PVP-coated superparamagnetic iron oxide. Contrast Media Mol Imaging. 2009; 4:118–126.6. Wang L, Deng J, Wang J, Xiang B, Yang T, Gruwel M, et al. Superparamagnetic iron oxide does not affect the viability and function of adipose-derived stem cells, and superparamagnetic iron oxide-enhanced magnetic resonance imaging identifies viable cells. Magn Reson Imaging. 2009; 27:108–119.7. Byun JS, Kwak BK, Kim JK, Jung J, Ha BC, Park S. Engraftment of human mesenchymal stem cells in a rat photothrombotic cerebral infarction model: comparison of intra-arterial and intravenous infusion using MRI and histological analysis. J Korean Neurosurg Soc. 2013; 54:467–476.8. Ha BC, Jung J, Kwak BK. Susceptibility-weighted imaging for stem cell visualization in a rat photothrombotic cerebral infarction model. Acta Radiol. 2015; 56:219–227.9. Xu HS, Ma C, Cao L, Wang JJ, Fan XX. Study of co-transplantation of SPIO labeled bone marrow stromal stem cells and Schwann cells for treating traumatic brain injury in rats and in vivo tracing of magnetically labeled cells by MRI. Eur Rev Med Pharmacol Sci. 2014; 18:520–525.10. Detante O, Valable S, de Fraipont F, Grillon E, Barbier EL, Moisan A, et al. Magnetic resonance imaging and fluorescence labeling of clinical-grade mesenchymal stem cells without impacting their phenotype: study in a rat model of stroke. Stem Cells Transl Med. 2012; 1:333–341.11. Vasconcelos-dos-Santos A, Rosado-de-Castro PH, Lopes de Souza SA, da Costa Silva J, Ramos AB, Rodriguez de Freitas G, et al. Intravenous and intra-arterial administration of bone marrow mononuclear cells after focal cerebral ischemia: is there a difference in biodistribution and efficacy? Stem Cell Res. 2012; 9:1–8.12. Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG, et al. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008; 39:1569–1574.13. Johnson GA, Calabrese E, Badea A, Paxinos G, Watson C. A multidimensional magnetic resonance histology atlas of the Wistar rat brain. Neuroimage. 2012; 62:1848–1856.14. Seki F, Hikishima K, Nambu S, Okanoya K, Okano HJ, Sasaki E, et al. Multidimensional MRI-CT atlas of the naked mole-rat brain (Heterocephalus glaber). Front Neuroanat. 2013; 7:45.15. Daadi MM, Li Z, Arac A, Grueter BA, Sofilos M, Malenka RC, et al. Molecular and magnetic resonance imaging of human embryonic stem cell-derived neural stem cell grafts in ischemic rat brain. Mol Ther. 2009; 17:1282–1291.16. Jung J, Kwak BK, Reddy AM, Ha BC, Shim HJ, Byun JS, et al. Characterization of photothrombotic cerebral infarction model at sensorimotor area of functional map in rat. J Neurol Sci-Turk. 2013; 30:617–628.17. Shen LH, Li Y, Chen J, Zhang J, Vanguri P, Borneman J, et al. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience. 2006; 137:393–399.18. Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003; 92:692–699.19. Guzman R, Choi R, Gera A, De Los Angeles A, Andres RH, Steinberg GK. Intravascular cell replacement therapy for stroke. Neurosurg Focus. 2008; 24:E15.20. Pendharkar AV, Chua JY, Andres RH, Wang N, Gaeta X, Wang H, et al. Biodistribution of neural stem cells after intravascular therapy for hypoxic-ischemia. Stroke. 2010; 41:2064–2070.21. Lundberg J, Le Blanc K, Söderman M, Andersson T, Holmin S. Endovascular transplantation of stem cells to the injured rat CNS. Neuroradiology. 2009; 51:661–667.22. Li L, Jiang Q, Ding G, Zhang L, Zhang ZG, Li Q, et al. Effects of administration route on migration and distribution of neural progenitor cells transplanted into rats with focal cerebral ischemia, an MRI study. J Cereb Blood Flow Metab. 2010; 30:653–662.23. Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009; 18:683–692.24. Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007; 40:609–619.25. Cheng JL, Yang YJ, Li HL, Wang J, Wang MH, Zhang Y. In vivo tracing of superparamagnetic iron oxide-labeled bone marrow mesenchymal stem cells transplanted for traumatic brain injury by susceptibility weighted imaging in a rat model. Chin J Traumatol. 2010; 13:173–177.26. Jülke H, Veit C, Ribitsch I, Brehm W, Ludewig E, Delling U. Comparative labelling of equine and ovine multipotent stromal cells with superparamagnetic iron oxide particles for magnetic resonance imaging in vitro. Cell Transplant. 2013; 12. 10. [Epub].27. Pawelczyk E, Arbab AS, Chaudhry A, Balakumaran A, Robey PG, Frank JA. In vitro model of bromodeoxyuridine or iron oxide nanoparticle uptake by activated macrophages from labeled stem cells: implications for cellular therapy. Stem Cells. 2008; 26:1366–1375.28. Cohen ME, Muja N, Fainstein N, Bulte JW, Ben-Hur T. Conserved fate and function of ferumoxides-labeled neural precursor cells in vitro and in vivo. J Neurosci Res. 2010; 88:936–944.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Engraftment of Human Mesenchymal Stem Cells in a Rat Photothrombotic Cerebral Infarction Model : Comparison of Intra-Arterial and Intravenous Infusion Using MRI and Histological Analysis
- Comparison of Superparamagnetic Iron Oxide Labeling Efficiency between Poly-L-Lysine and Protamine Sulfate for Human Mesenchymal Stem Cells: Quantitative Analysis Using Multi-Echo T2* Magnetic Resonance Imaging
- In vivo Tracking of Mesenchymal Stem Cells Labeled with a Novel Chitosan-coated Superparamagnetic Iron Oxide Nanoparticles using 3.0T MRI
- Effects of Endothelial Progenitor Cells Used for Autograft Transplantation in Acute Myocardial Infarction Pig Model
- Monitoring Transplanted Human Mesenchymal Stem Cells in the Penile Cavernosal Tissues of Streptozotocin-induced Diabetic Rats Using Molecular Magnetic Resonance Imaging