J Korean Soc Radiol.
2013 Feb;68(2):175-185. 10.3348/jksr.2013.68.2.175.
Comparison of Superparamagnetic Iron Oxide Labeling Efficiency between Poly-L-Lysine and Protamine Sulfate for Human Mesenchymal Stem Cells: Quantitative Analysis Using Multi-Echo T2* Magnetic Resonance Imaging
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea. jeonghlee@hanmir.com
- 2MRI Team, Korea Basic Science Institute, Daejeon, Korea.
- KMID: 2097996
- DOI: http://doi.org/10.3348/jksr.2013.68.2.175
Abstract
- PURPOSE
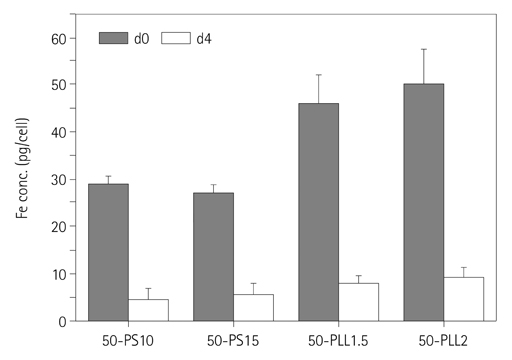
To quantify in vitro labeling efficiency of protamine sulfate (PS) and poly-L-lysine (PLL) for labeling of human mesenchymal stem cells (hMSCs) with superparamagnetic iron oxide (SPIO) using multi-echo T2* magnetic resonance (MR) imaging at 4.7 T.
MATERIALS AND METHODS
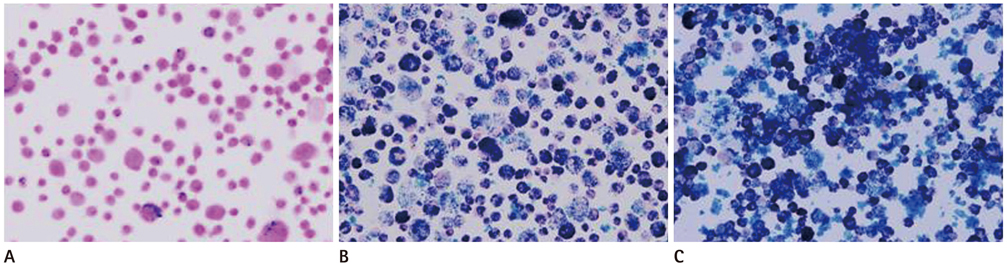
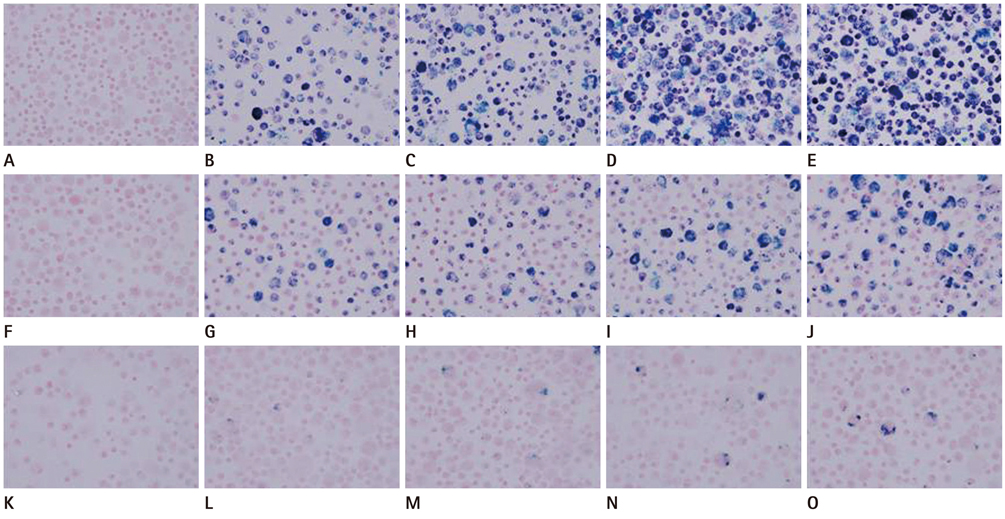
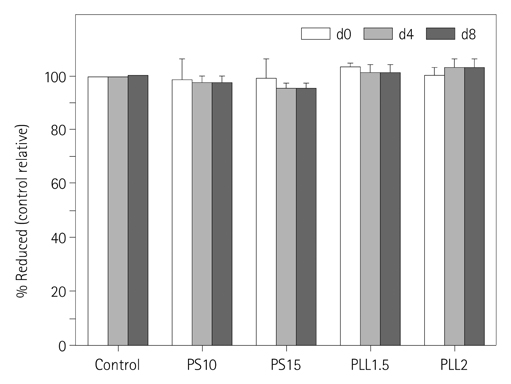
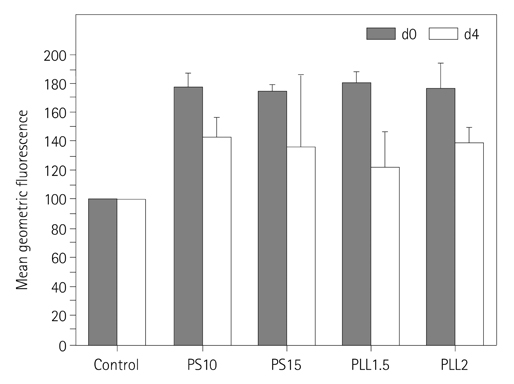
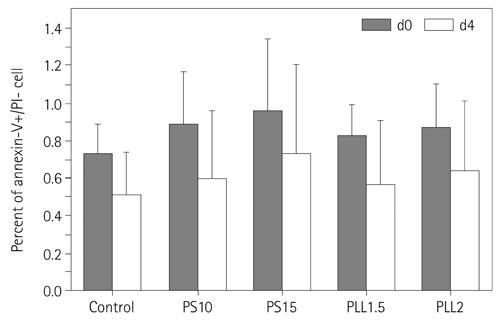
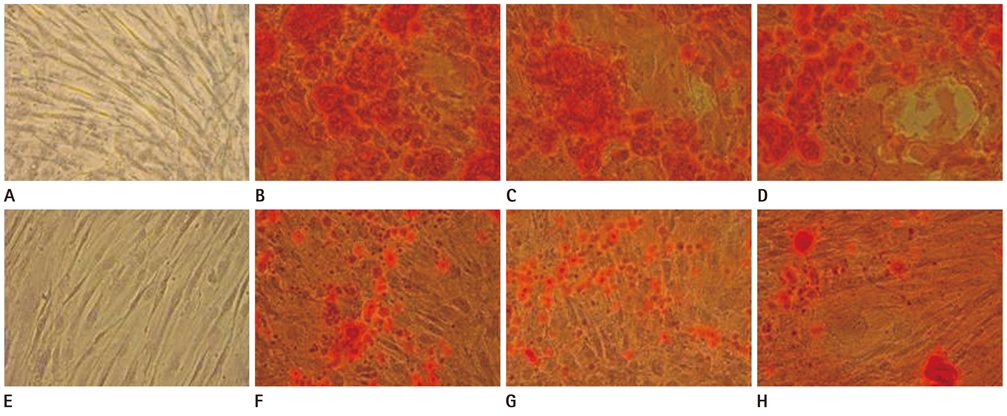
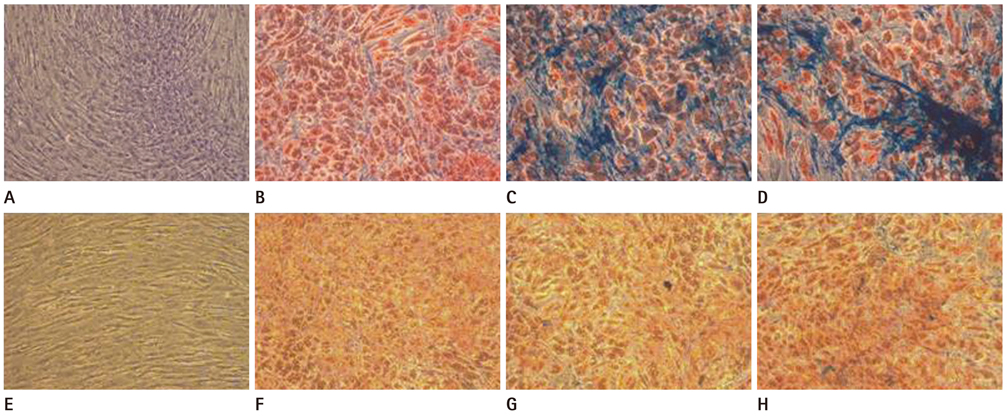
The hMSCs were incubated with SPIO-PS or SPIO-PLL complexes. Their effects on the cell metabolism and differentiation capability were evaluated, respectively. The decrease of iron concentrations in the labeled cells were assessed immediately, and at 4 d after labeling using multi-echo T2* MR imaging at 4.7 T. The results were compared with those of Prussian blue colorimetry.
RESULTS
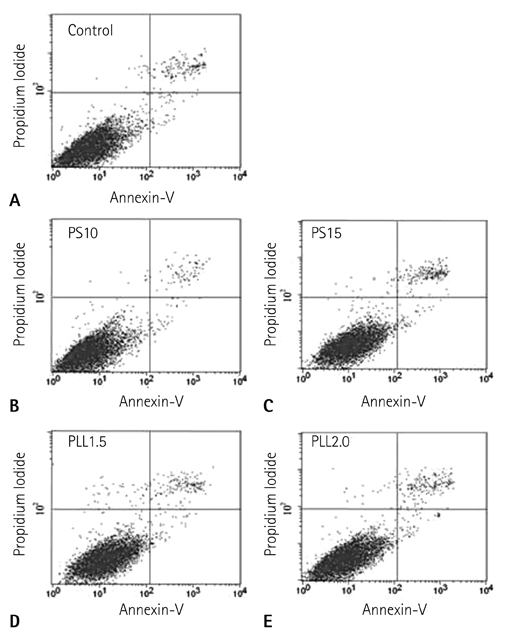
The hMSCs were labeled more efficiently by SPIO-PLL than SPIO-PS without any significant effect on cell metabolism and differentiation capabilities. It was feasible to quantify the iron concentrations in SPIO-agarose-phantoms and in agarose mixture with the labeled cells from T2* maps obtained from multi-echo T2* MRI. However, the iron concentration of the labeled cells was significantly higher by T2*-maps than the results of Prussian blue colorimetry.
CONCLUSION
The hMSCs can be effectively labeled with SPIO-PLL complexes more than with SPIO-PS without significant change in cell metabolism and differentiation. In vitro quantification of the iron concentrations of the labeled is feasible from multi-echo T2* MRI, but needs further investigation.
MeSH Terms
Figure
Reference
-
1. Dazzi F, Horwood NJ. Potential of mesenchymal stem cell therapy. Curr Opin Oncol. 2007. 19:650–655.2. Serakinci N, Keith WN. Therapeutic potential of adult stem cells. Eur J Cancer. 2006. 42:1243–1246.3. Mowat P, Franconi F, Chapon C, Lemaire L, Dorat J, Hindré F, et al. Evaluating SPIO-labelled cell MR efficiency by three-dimensional quantitative T2* MRI. NMR Biomed. 2007. 20:21–27.4. Bulte JW, Duncan ID, Frank JA. In vivo magnetic resonance tracking of magnetically labeled cells after transplantation. J Cereb Blood Flow Metab. 2002. 22:899–907.5. Arbab AS, Yocum GT, Kalish H, Jordan EK, Anderson SA, Khakoo AY, et al. Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood. 2004. 104:1217–1223.6. Walczak P, Kedziorek DA, Gilad AA, Barnett BP, Bulte JW. Applicability and limitations of MR tracking of neural stem cells with asymmetric cell division and rapid turnover: the case of the shiverer dysmyelinated mouse brain. Magn Reson Med. 2007. 58:261–269.7. Oude Engberink RD, van der Pol SM, Döpp EA, de Vries HE, Blezer EL. Comparison of SPIO and USPIO for in vitro labeling of human monocytes: MR detection and cell function. Radiology. 2007. 243:467–474.8. Oppitz M, Pintaske J, Kehlbach R, Schick F, Schriek G, Busch C. Magnetic resonance imaging of iron-oxide labeled SK-Mel 28 human melanoma cells in the chick embryo using a clinical whole body MRI scanner. MAGMA. 2007. 20:1–9.9. Ju S, Teng GJ, Lu H, Zhang Y, Zhang A, Chen F, et al. In vivo MR tracking of mesenchymal stem cells in rat liver after intrasplenic transplantation. Radiology. 2007. 245:206–215.10. Ju S, Teng G, Zhang Y, Ma M, Chen F, Ni Y. In vitro labeling and MRI of mesenchymal stem cells from human umbilical cord blood. Magn Reson Imaging. 2006. 24:611–617.11. Hauger O, Frost EE, van Heeswijk R, Deminière C, Xue R, Delmas Y, et al. MR evaluation of the glomerular homing of magnetically labeled mesenchymal stem cells in a rat model of nephropathy. Radiology. 2006. 238:200–210.12. Unger EC. How can superparamagnetic iron oxides be used to monitor disease and treatment? Radiology. 2003. 229:615–616.13. Frank JA, Miller BR, Arbab AS, Zywicke HA, Jordan EK, Lewis BK, et al. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003. 228:480–487.14. Arbab AS, Bashaw LA, Miller BR, Jordan EK, Lewis BK, Kalish H, et al. Characterization of biophysical and metabolic properties of cells labeled with superparamagnetic iron oxide nanoparticles and transfection agent for cellular MR imaging. Radiology. 2003. 229:838–846.15. Bos C, Delmas Y, Desmoulière A, Solanilla A, Hauger O, Grosset C, et al. In vivo MR imaging of intravascularly injected magnetically labeled mesenchymal stem cells in rat kidney and liver. Radiology. 2004. 233:781–789.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of Optimal Combination of Commercially Available Superparamagnetic Iron Oxide Nanoparticles and Transfection Agents for Labelling of Human Mesenchymal Stem Cells
- Labeling Efficacy of Superparamagnetic Iron Oxide Nanoparticles to Human Neural Stem Cells: Comparison of Ferumoxides, Monocrystalline Iron Oxide, Cross-linked Iron Oxide (CLIO)-NH2 and tat-CLIO
- Histologic Monitoring of the Transplanted Superparamagnetic Iron Oxide Labelled Human Mesenchymal Stem Cells in the Rat Bladder
- In vivo Tracking of Mesenchymal Stem Cells Labeled with a Novel Chitosan-coated Superparamagnetic Iron Oxide Nanoparticles using 3.0T MRI
- Evaluation of Engraftment of Superparamagnetic Iron Oxide-Labeled Mesenchymal Stem Cells Using Three-Dimensional Reconstruction of Magnetic Resonance Imaging in Photothrombotic Cerebral Infarction Models of Rats