Korean J Urol.
2015 Apr;56(4):305-309. 10.4111/kju.2015.56.4.305.
Significance of intraprostatic architecture and regrowth velocity for considering discontinuation of dutasteride after combination therapy with an alpha blocker: A prospective, pilot study
- Affiliations
-
- 1Department of Urology, NTT East Corporation Sapporo Hospital, Sapporo, Japan. shindo1013@yahoo.co.jp
- 2Department of Urology, Sapporo Medical University School of Medicine, Sapporo, Japan.
- KMID: 2155306
- DOI: http://doi.org/10.4111/kju.2015.56.4.305
Abstract
- PURPOSE
We conducted a prospective single-center study to evaluate the possibility of discontinuation of dutasteride after combination therapy with an alpha blocker for benign prostatic hyperplasia (BPH).
MATERIALS AND METHODS
We prospectively treated BPH patients with an alpha blocker and dutasteride (0.5 mg/d). Patients who had been treated with alpha blockers against BPH for more than 2 months were eligible, and 20 patients were included in the study. After 6 months of combination therapy, dutasteride was discontinued. Patients were followed for 12 months after cessation. Prostate volume, intraprostatic architecture determined by transrectal ultrasound, peak urinary flow rate, postvoid residual urine volume, and the serum prostate-specific antigen level were evaluated every 6 months, and the International Prostate Symptom Score and overactive bladder symptom score (OABSS) every 3 months. Patients were allowed to restart dutasteride during the follow-up period according to their desire.
RESULTS
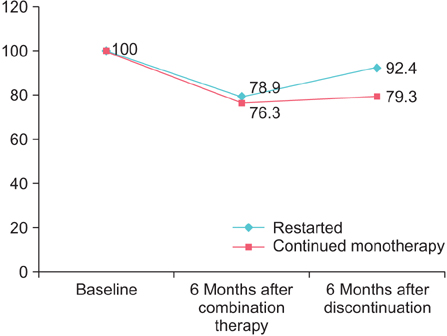
Twelve patients (12/20, 60%) restarted the combination therapy from 6 to 12 months into the follow-up period. For patients who restarted dutasteride, the prostate volume and OABSS had increased and worsened after discontinuation, respectively. A visible transition zone with a clear border on transrectal ultrasound at baseline and regrowth of the prostate after discontinuation of dutasteride were risk factors for restarting the therapy (Mann-Whitney U test: p=0.008, p=0.017).
CONCLUSIONS
Prostatic enlargement after discontinuation of dutasteride differs among patients. Rapid regrowth of the prostate leads to deterioration of storage symptoms and a tendency to restart dutasteride. Baseline intraprostatic architecture may be a predictive factor for whether the patient is a good candidate for discontinuation.
MeSH Terms
-
5-alpha Reductase Inhibitors/administration & dosage/adverse effects
*Adrenergic alpha-Antagonists/administration & dosage/adverse effects
Aged
Drug Monitoring
Drug Therapy, Combination/methods
*Dutasteride/administration & dosage/adverse effects
Follow-Up Studies
Humans
Japan
Male
Middle Aged
Organ Size
Prospective Studies
*Prostate/drug effects/pathology/ultrasonography
Prostate-Specific Antigen/analysis
*Prostatic Hyperplasia/drug therapy/pathology
Secondary Prevention/methods/statistics & numerical data
Treatment Outcome
Withholding Treatment
5-alpha Reductase Inhibitors
Adrenergic alpha-Antagonists
Dutasteride
Prostate-Specific Antigen
Figure
Reference
-
1. Wu XJ, Zhi Y, Zheng J, He P, Zhou XZ, Li WB, et al. Dutasteride on benign prostatic hyperplasia: a meta-analysis on randomized clinical trials in 6460 patients. Urology. 2014; 83:539–543.2. Roehrborn CG, Barkin J, Siami P, Tubaro A, Wilson TH, Morrill BB, et al. Clinical outcomes after combined therapy with dutasteride plus tamsulosin or either monotherapy in men with benign prostatic hyperplasia (BPH) by baseline characteristics: 4-year results from the randomized, double-blind Combination of Avodart and Tamsulosin (CombAT) trial. BJU Int. 2011; 107:946–954.3. Roehrborn CG, Barkin J, Tubaro A, Emberton M, Wilson TH, Brotherton BJ, et al. Influence of baseline variables on changes in International Prostate Symptom Score after combined therapy with dutasteride plus tamsulosin or either monotherapy in patients with benign prostatic hyperplasia and lower urinary tract symptoms: 4-year results of the CombAT study. BJU Int. 2014; 113:623–635.4. Masumori N, Tsukamoto T, Rhodes T, Girman CJ. Natural history of lower urinary tract symptoms in men: result of a longitudinal community-based study in Japan. Urology. 2003; 61:956–960.5. Barkin J, Guimaraes M, Jacobi G, Pushkar D, Taylor S, van Vierssen Trip OB. Alpha-blocker therapy can be withdrawn in the majority of men following initial combination therapy with the dual 5alpha-reductase inhibitor dutasteride. Eur Urol. 2003; 44:461–466.6. Fukuta F, Masumori N, Mori M, Tsukamoto T. Internal prostatic architecture on transrectal ultrasonography predicts future prostatic growth: natural history of prostatic hyperplasia in a 15-year longitudinal community-based study. Prostate. 2011; 71:597–603.7. Homma Y, Yoshida M, Seki N, Yokoyama O, Kakizaki H, Gotoh M, et al. Symptom assessment tool for overactive bladder syndrome: overactive bladder symptom score. Urology. 2006; 68:318–323.8. Jeong YB, Kwon KS, Kim SD, Kim HJ. Effect of discontinuation of 5alpha-reductase inhibitors on prostate volume and symptoms in men with BPH: a prospective study. Urology. 2009; 73:802–806.9. Lee JY, Kang DH, Park SY, Lee SW, Kim YT, Choi HY. Effect of discontinuation of tamusulosin in Korean men with benign prostatic hyperplasia taking tamusulosin and dutasteride: An open-label prospective, randomized pilot study. LUTS. 2012; 4:35–40.10. Yoshimura K, Ohara H, Ichioka K, Terada N, Matsui Y, Terai A, et al. Nocturia and benign prostatic hyperplasia. Urology. 2003; 61:786–790.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Effects of Discontinuing 5-Alpha Reductase Inhibitor in Patients With Benign Prostatic Hyperplasia
- A Case of Combination Therapy with Finasteride and Low Dose Dutasteride in the Treatment of Androgenetic Alopecia
- The Change of Prostate-specific Antigen and Prostate-specific Antigen Density in Patients with Benign Prostatic Hyperplasia after Dutasteride Treatment
- alpha-Blocker Monotherapy and alpha-Blocker Plus 5-Alpha-Reductase Inhibitor Combination Treatment in Benign Prostatic Hyperplasia; 10 Years' Long-Term Results
- Comparison of Clinical Efficacy of Finasteride and Dutasteride as 5-alpha Reductase Inhibitor