Ewha Med J.
2016 Jan;39(1):23-27. 10.12771/emj.2016.39.1.23.
Tocilizumab-induced Transaminitis in a Seropositive Rheumatoid Arthritis Patient with Macrophage Activation Syndrome
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. sangwonlee@yuhs.ac
- KMID: 2152754
- DOI: http://doi.org/10.12771/emj.2016.39.1.23
Abstract
- As a new humanized monoclonal antibody against the interleukin-6 receptor, tocilizumab is currently used for the treatment of rheumatoid arthritis (RA) patients. Tocilizumab was reported to provoke drug-related liver toxicity, although there have been no reports on significant liver toxicity from tocilizumab in Korean patients with RA to date. Here, we describe the first case of tocilizumab-related liver toxicity in a patient with complicated RA, accompanied with macrophage activation syndrome, who had received tacrolimus and prednisolone and in whom both conventional disease modifying anti-rheumatic drugs, including methotrexate, leflunomide and sulfasalazine or tumor necrotizing factor-alpha blockades, were contraindicated due to drug eruption and a history of lung cancer.
Keyword
MeSH Terms
-
Antirheumatic Agents
Arthritis, Rheumatoid*
Drug Eruptions
Humans
Interleukin-6
Liver
Lung Neoplasms
Macrophage Activation Syndrome*
Macrophage Activation*
Macrophages*
Methotrexate
Prednisolone
Sulfasalazine
Tacrolimus
Tranexamic Acid*
Antirheumatic Agents
Interleukin-6
Methotrexate
Prednisolone
Sulfasalazine
Tacrolimus
Tranexamic Acid
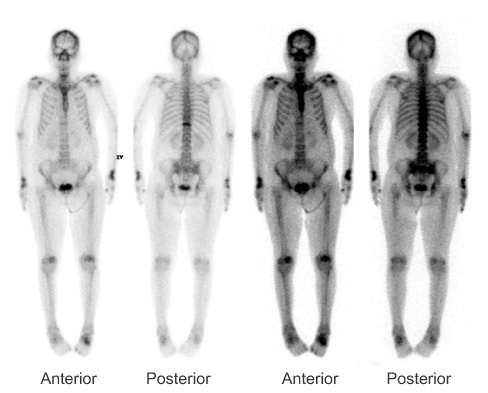
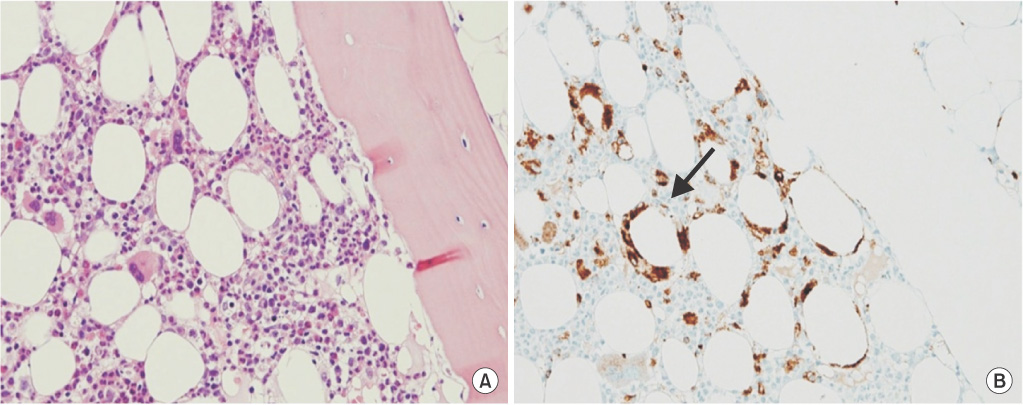
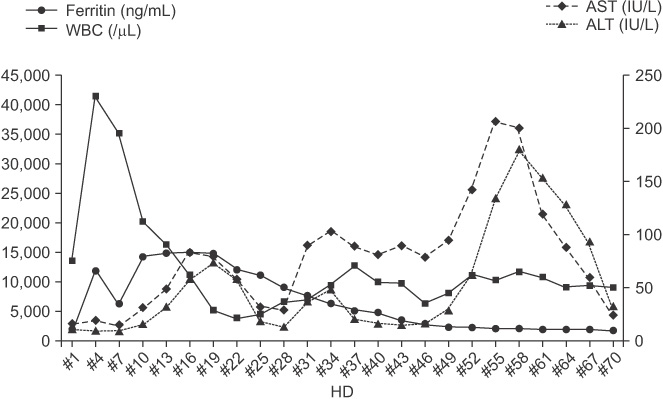
Figure
Reference
-
1. Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008; 67:1516–1523.2. Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008; 58:2968–2980.3. Maini RN, Taylor PC, Szechinski J, Pavelka K, Broll J, Balint G, et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006; 54:2817–2829.4. Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez-Reino JJ, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010; 69:88–96.5. Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008; 371:987–997.6. Ravelli A, Magni-Manzoni S, Pistorio A, Besana C, Foti T, Ruperto N, et al. Preliminary diagnostic guidelines for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. J Pediatr. 2005; 146:598–604.7. Alfreijat M, Habibi M, Bhatia P, Bhatia A. Severe hepatitis associated with tocilizumab in a patient with rheumatoid arthritis. Rheumatology (Oxford). 2013; 52:1340–1341.8. Mahamid M, Paz K, Reuven M, Safadi R. Hepatotoxicity due to tocilizumab and anakinra in rheumatoid arthritis: two case reports. Int J Gen Med. 2011; 4:657–660.9. Kovalovich K, DeAngelis RA, Li W, Furth EE, Ciliberto G, Taub R. Increased toxin-induced liver injury and fibrosis in interleukin-6-deficient mice. Hepatology. 2000; 31:149–159.10. Drepper M, Rubbia-Brandt L, Spahr L. Tocilizumab-induced acute liver injury in adult onset Still's disease. Case Reports Hepatol. 2013; 2013:964828.11. Schiff MH, Kremer JM, Jahreis A, Vernon E, Isaacs JD, van Vollenhoven RF. Integrated safety in tocilizumab clinical trials. Arthritis Res Ther. 2011; 13:R141.12. Kobayashi M, Takahashi Y, Yamashita H, Kaneko H, Mimori A. Benefit and a possible risk of tocilizumab therapy for adult-onset Still's disease accompanied by macrophage-activation syndrome. Mod Rheumatol. 2011; 21:92–96.13. Deane S, Selmi C, Teuber SS, Gershwin ME. Macrophage activation syndrome in autoimmune disease. Int Arch Allergy Immunol. 2010; 153:109–120.14. Arico M, Danesino C, Pende D, Moretta L. Pathogenesis of haemophagocytic lymphohistiocytosis. Br J Haematol. 2001; 114:761–769.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Sepsis Caused by Cellulitis in a Patient with Rheumatoid Arthritis after Tocilizumab Treatment
- Drug retention of biologic and targeted synthetic disease-modifying antirheumatic drugs in Korean patients with seropositive rheumatoid arthritis
- Acute Ischemic Stroke in the Patients with Inflammatory Arthritis: An Analysis of Data from National Health Insurance Service
- Macrophage Activation Syndrome in Juvenile Rheumatoid Arthritis Successfully Treated with Cyclosporine A: A Case Report
- A Case of Gold Induced Hypersensitivity Pneumonitis Diagnosed by Lymphocyte Stimulation Test with Gold