J Korean Med Sci.
2014 Nov;29(Suppl 3):S217-S221. 10.3346/jkms.2014.29.S3.S217.
Efficacy and Safety of Porcine Collagen Filler for Nasolabial Fold Correction in Asians: A Prospective Multicenter, 12 Months Follow-up Study
- Affiliations
-
- 1Department of Plastic and Reconstructive Surgery, The Catholic University of Korea, Seoul, Korea. joony@catholic.ac.kr
- KMID: 2151416
- DOI: http://doi.org/10.3346/jkms.2014.29.S3.S217
Abstract
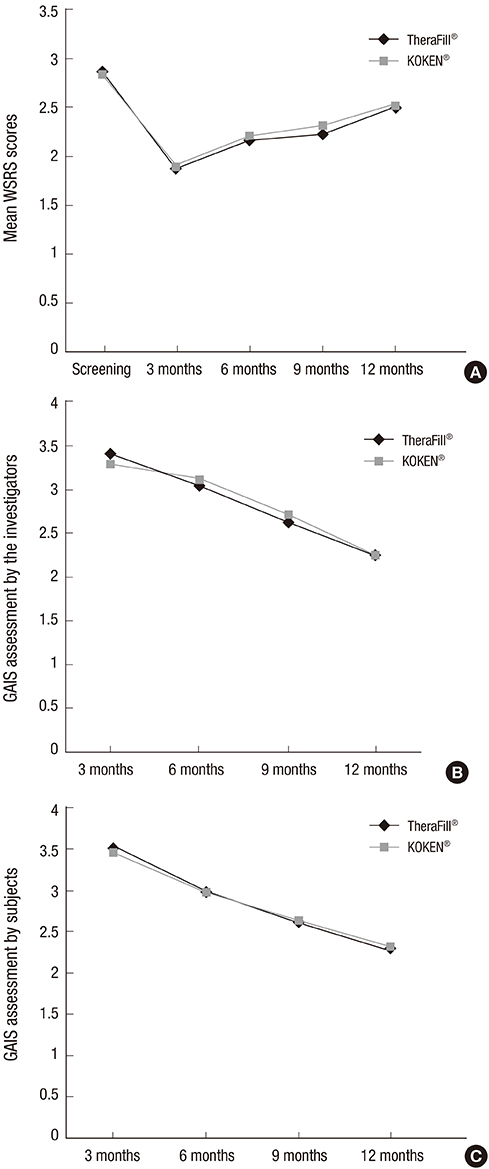
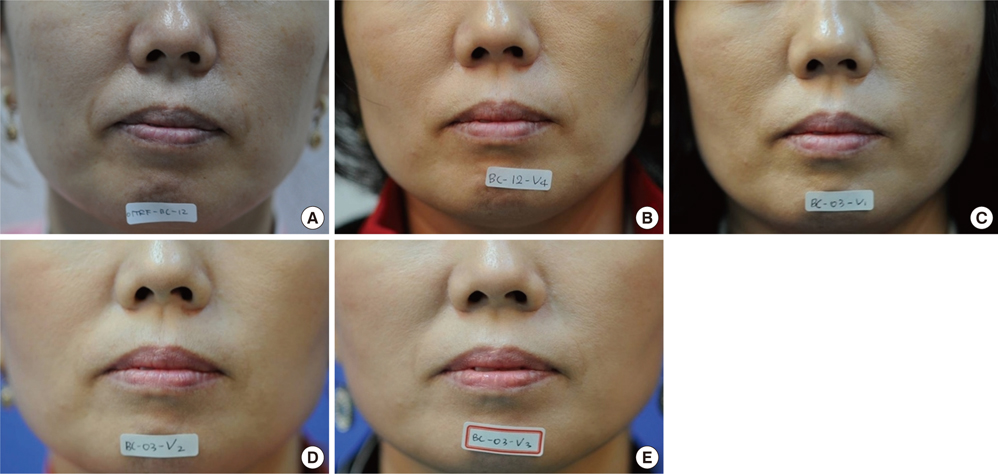
- Recently, injectable dermal fillers have become important alternatives to surgical procedures for the correction of facial wrinkles. Bovine collagen is the first approved material for filler injection, and several studies have shown its efficacy. However, the risk of developing an allergic reaction and xenogenic transmission of bovine spongiform encephalopathy remain among its disadvantages. In this randomized, double-blinded, split-face study, we compared the efficacy and safety of a porcine collagen filler (TheraFill(R)) with that of a bovine collagen filler (KOKEN(R)) for nasolabial fold correction. A total of sixty one patients with mild to severe nasolabial fold were randomized to receive TheraFill(R) and KOKEN(R) on contralateral sides of the face. During the 12-month follow-up period, improvement in the Wrinkle-Severity Rating Scale score was slightly higher in TheraFill(R) group than KOKEN(R) group, although the difference was not statistically significant. No serious adverse reactions were observed and both materials were tolerable in most cases. In conclusion, the long-term effect of TheraFill(R) on nasolabial fold correction was comparable to that of KOKEN(R), and it may be a good alternative to bovine collagen filler.
Keyword
MeSH Terms
-
Adult
Aged
Animals
Biocompatible Materials/therapeutic use
Cattle
Collagen/adverse effects/*therapeutic use
Dermatologic Surgical Procedures/*methods
Double-Blind Method
Face/surgery
Female
Follow-Up Studies
Humans
Injections, Intradermal
Male
Middle Aged
Nasolabial Fold/*surgery
Prospective Studies
Skin Aging
Surgery, Plastic/*methods
Swine
Treatment Outcome
Biocompatible Materials
Collagen
Figure
Reference
-
1. Fenske NA, Lober CW. Structural and functional changes of normal aging skin. J Am Acad Dermatol. 1986; 15:571–585.2. Vidal P, Berner JE, Castillo P, Rochefort G, Loubies R. Descended mouth corner: an ignored but needed feature of facial rejuvenation. Arch Plast Surg. 2013; 40:783–786.3. Lee JW, Cho BC, Lee KY. Direct brow lift combined with suspension of the orbicularis oculi muscle. Arch Plast Surg. 2013; 40:603–609.4. Lee SY, Sung KY. Subcision using a spinal needle cannula and a thread for prominent nasolabial fold correction. Arch Plast Surg. 2013; 40:256–258.5. Lee SK, Kim DW, Dhong ES, Park SH, Yoon ES. Facial soft tissue augmentation using autologous fat mixed with stromal vascular fraction. Arch Plast Surg. 2012; 39:534–539.6. Salibian AA, Widgerow AD, Abrouk M, Evans GR. Stem cells in plastic surgery: a review of current clinical and translational applications. Arch Plast Surg. 2013; 40:666–675.7. Shin KC, Bae TH, Kim WS, Kim HK. Usefulness of gold thread implantation for crow's feet. Arch Plast Surg. 2012; 39:42–45.8. Kanchwala SK, Holloway L, Bucky LP. Reliable soft tissue augmentation: a clinical comparison of injectable soft-tissue fillers for facial-volume augmentation. Ann Plast Surg. 2005; 55:30–35. discussion 5.9. Kaufman MR, Miller TA, Huang C, Roostaeian J, Wasson KL, Ashley RK, Bradley JP. Autologous fat transfer for facial recontouring: is there science behind the art? Plast Reconstr Surg. 2007; 119:2287–2296.10. Murray CA, Zloty D, Warshawski L. The evolution of soft tissue fillers in clinical practice. Dermatol Clin. 2005; 23:343–363.11. Watson W, Kaye RL, Klein A, Stegman S. Injectable collagen: a clinical overview. Cutis. 1983; 31:543–546.12. Baumann L, Kaufman J, Saghari S. Collagen fillers. Dermatol Ther. 2006; 19:134–140.13. Andre P, Lowe NJ, Parc A, Clerici TH, Zimmermann U. Adverse reactions to dermal fillers: a review of European experiences. J Cosmet Laser Ther. 2005; 7:171–176.14. Lavker RM. Structural alterations in exposed and unexposed aged skin. J Invest Dermatol. 1979; 73:59–66.15. Griffiths CE, Russman AN, Majmudar G, Singer RS, Hamilton TA, Voorhees JJ. Restoration of collagen formation in photodamaged human skin by tretinoin (retinoic acid). N Engl J Med. 1993; 329:530–535.16. Cockerham K, Hsu VJ. Collagen-based dermal fillers: past, present, future. Facial Plast Surg. 2009; 25:106–113.17. Klein AW, Elson ML. The history of substances for soft tissue augmentation. Dermatol Surg. 2000; 26:1096–1105.18. Downie J, Mao Z, Rachel Lo TW, Barry S, Bock M, Siebert JP, Bowman A, Ayoub A. A double-blind, clinical evaluation of facial augmentation treatments: a comparison of PRI 1, PRI 2, Zyplast and Perlane. J Plast Reconstr Aesthet Surg. 2009; 62:1636–1643.19. Patel KM, Nahabedian MY, Gatti M, Bhanot P. Indications and outcomes following complex abdominal reconstruction with component separation combined with porcine acellular dermal matrix reinforcement. Ann Plast Surg. 2012; 69:394–398.20. Alperin M. Collagen scaffold: a treatment for large mesh exposure following vaginal prolapse repair. Int Urogynecol J. 2014.21. Giannotti S, Ghilardi M, Dell'osso G, Magistrelli L, Bugelli G, Di Rollo F, Ricci G, Calabrese R, Siciliano G, Guido G. Study of the porcine dermal collagen repair patch in morpho-functional recovery of the rotator cuff after minimum follow-up of 2.5 Years. Surg Technol Int. 2014; 24:348–352.22. Narins RS, Brandt FS, Lorenc ZP, Maas CS, Monheit GD, Smith SR. Twelve-month persistency of a novel ribose-cross-linked collagen dermal filler. Dermatol Surg. 2008; 34:S31–S39.23. Goldberg DJ. Correction of tear trough deformity with novel porcine collagen dermal filler (Dermicol-P35). Aesthet Surg J. 2009; 29:S9–S11.24. Lorenc ZP, Nir E, Azachi M. Characterization of physical properties and histologic evaluation of injectable Dermicol-p35 porcine-collagen dermal filler. Plast Reconstr Surg. 2010; 125:1805–1813.25. Braun M, Braun S. Nodule formation following lip augmentation using porcine collagen-derived filler. J Drugs Dermatol. 2008; 7:579–581.26. Klein AW. Skin filling: Collagen and other injectables of the skin. Dermatol Clin. 2001; 19:491–508. ix27. Narins RS, Brandt F, Leyden J, Lorenc ZP, Rubin M, Smith S. A randomized, double-blind, multicenter comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds. Dermatol Surg. 2003; 29:588–595.28. Monstrey SJ, Pitaru S, Hamdi M, Van Landuyt K, Blondeel P, Shiri J, Goldlust A, Shoshani D. A two-stage phase I trial of Evolence30 collagen for soft-tissue contour correction. Plast Reconstr Surg. 2007; 120:303–311.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Randomized, Evaluator-Blinded, Split-Face Comparison Study of the Efficacy and Safety of a Novel Mannitol Containing Monophasic Hyaluronic Acid Dermal Filler for the Treatment of Moderate to Severe Nasolabial Folds
- Autologous fat injection and collagen impantation for the correction of facial atroply
- Efficacy of Retrobulbar Filler Injections for Correcting Enophthalmos Associated with Phthisis Bulbi or Anophthalmos
- Efficacy and Safety of Nasolabial Fold Correction Using Collagen-Stimulating Filler: A Systematic Review and Meta-Analysis
- The Correction of Deep Nasolabial Fold using Filling Material