Ann Dermatol.
2016 Jun;28(3):297-303. 10.5021/ad.2016.28.3.297.
A Randomized, Evaluator-Blinded, Split-Face Comparison Study of the Efficacy and Safety of a Novel Mannitol Containing Monophasic Hyaluronic Acid Dermal Filler for the Treatment of Moderate to Severe Nasolabial Folds
- Affiliations
-
- 1Department of Dermatology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. csesnumd@gmail.com gylee0716@daum.net
- 2Department of Dermatology, Hallym University Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- 3Bright and Clear Dermatology Clinic, Seoul, Korea.
- 4Department of Dermatology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. csesnumd@gmail.com gylee0716@daum.net
- KMID: 2164636
- DOI: http://doi.org/10.5021/ad.2016.28.3.297
Abstract
- BACKGROUND
Mannitol containing monophasic filler with higher crosslinking has not been well studied for moderate and severe nasolabial fold (NLF) correction.
OBJECTIVE
To compare the efficacy and safety of a novel mannitol containing hyaluronic acid (HA) filler (HA-G) with biphasic HA filler (HA-P) for moderate and severe NLF correction.
METHODS
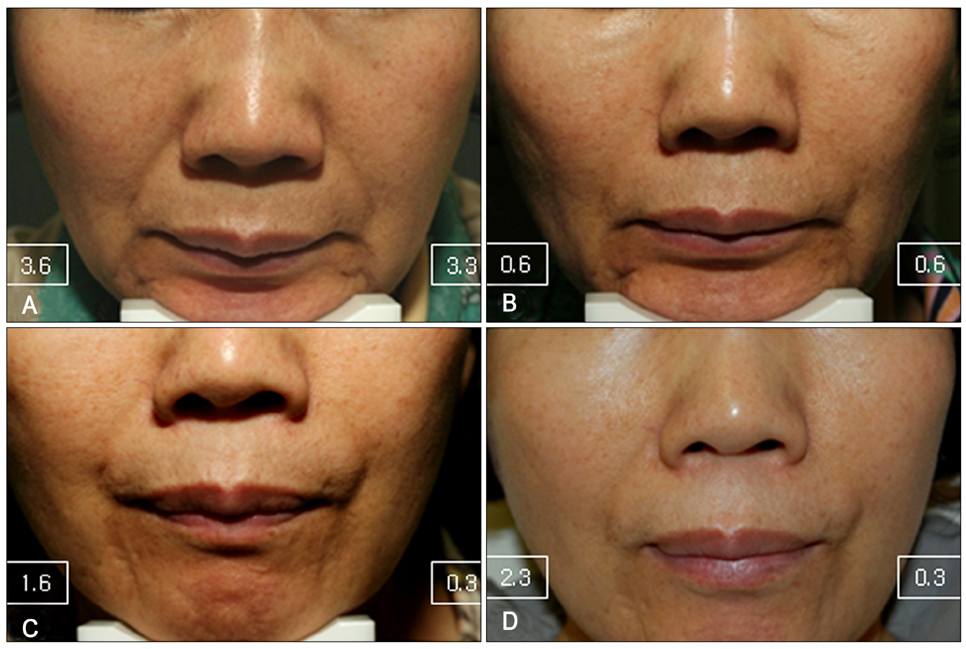
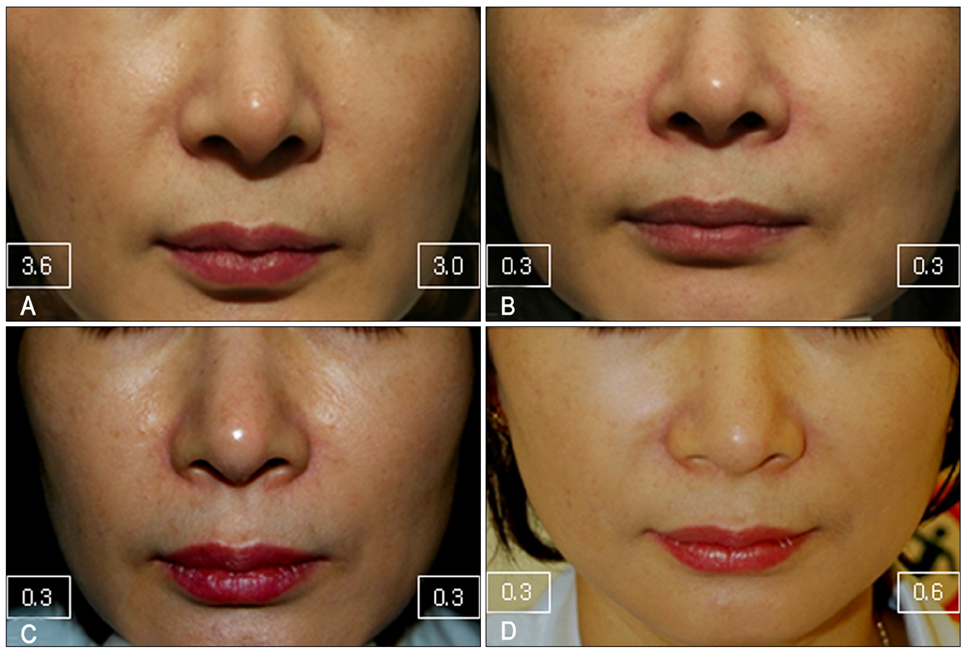
Thirteen subjects with symmetric moderate to severe NLF received HA-G (in one NLF) and HA-P (in other NLF) and were evaluated for 24 weeks.
RESULTS
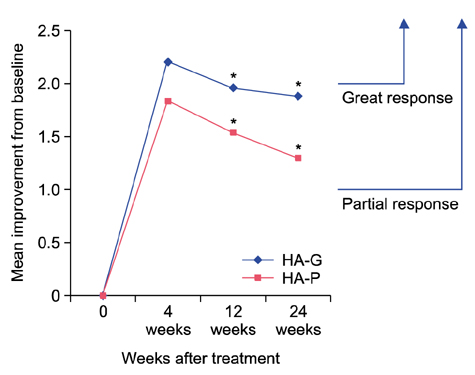
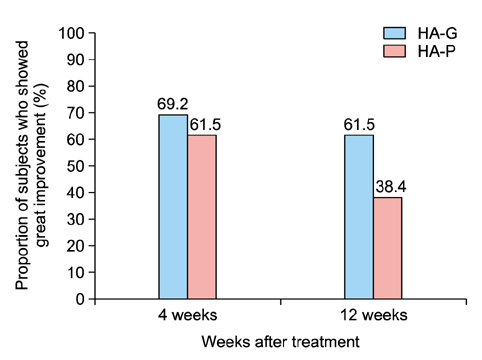
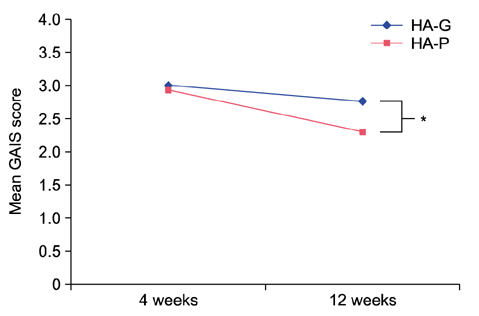
At both 12 and 24 weeks, the mean improvement in Genzyme 6-point grading scale from baseline was significantly greater in the side of face that was treated with HA-G than HA-P (1.96±0.91 vs. 1.54±0.73 at week 12; p=0.044, 1.88±0.78 vs. 1.3±0.79 at week 24; p=0.027, respectively). At 12 weeks, the mean Global Aesthetic Improvement Scale score was 2.92±0.93 for HA-G and 2.31±0.95 for HA-P (p=0.008). Both fillers were well tolerated.
CONCLUSION
The HA filler HA-G provides better efficacy and similar local tolerability compared with HA-P in 6 months following treatment for moderate and severe NLF.
Keyword
MeSH Terms
Figure
Reference
-
1. Kim H, Park KY, Choi SY, Koh HJ, Park SY, Park WS, et al. The efficacy, longevity, and safety of combined radiofrequency treatment and hyaluronic acid filler for skin rejuvenation. Ann Dermatol. 2014; 26:447–456.
Article2. Gold M. The science and art of hyaluronic acid dermal filler use in esthetic applications. J Cosmet Dermatol. 2009; 8:301–307.
Article3. Andre P. Hyaluronic acid and its use as a "rejuvenation" agent in cosmetic dermatology. Semin Cutan Med Surg. 2004; 23:218–222.
Article4. Carruthers A, Carruthers J. Non-animal-based hyaluronic acid fillers: scientific and technical considerations. Plast Reconstr Surg. 2007; 120:33S–40S.
Article5. Wang F, Garza LA, Kang S, Varani J, Orringer JS, Fisher GJ, et al. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007; 143:155–163.
Article6. Ohrem HL, Schornick E, Kalivoda A, Ognibene R. Why is mannitol becoming more and more popular as a pharmaceutical excipient in solid dosage forms? Pharm Dev Technol. 2014; 19:257–262.
Article7. Andrews RJ, Bringas JR, Muto RP. Effects of mannitol on cerebral blood flow, blood pressure, blood viscosity, hematocrit, sodium, and potassium. Surg Neurol. 1993; 39:218–222.
Article8. Goodman GJ, Bekhor P, Rich M, Rosen RH, Halstead MB, Rogers JD. A comparison of the efficacy, safety, and longevity of two different hyaluronic acid dermal fillers in the treatment of severe nasolabial folds: a multicenter, prospective, randomized, controlled, single-blind, withinsubject study. Clin Cosmet Investig Dermatol. 2011; 4:197–205.
Article9. Ascher B, Bayerl C, Brun P, Kestemont P, Rzany B, Poncet M, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of severe nasolabial lines-6-month interim results of a randomized, evaluator-blinded, intra-individual comparison study. J Cosmet Dermatol. 2011; 10:94–98.
Article10. Nast A, Reytan N, Hartmann V, Pathirana D, Bachmann F, Erdmann R, et al. Efficacy and durability of two hyaluronic acid-based fillers in the correction of nasolabial folds: results of a prospective, randomized, double-blind, actively controlled clinical pilot study. Dermatol Surg. 2011; 37:768–775.
Article11. Monheit GD, Gendler EC, Poff B, Fleming L, Bachtell N, Garcia E, et al. Development and validation of a 6-point grading scale in patients undergoing correction of nasolabial folds with a collagen implant. Dermatol Surg. 2010; 36:Suppl 3. 1809–1816.
Article12. Edsman K, Nord LI, Ohrlund A, Larkner H, Kenne AH. Gel properties of hyaluronic acid dermal fillers. Dermatol Surg. 2012; 38:1170–1179.
Article13. Falcone SJ, Berg RA. Crosslinked hyaluronic acid dermal fillers: a comparison of rheological properties. J Biomed Mater Res A. 2008; 87:264–271.
Article14. Kablik J, Monheit GD, Yu L, Chang G, Gershkovich J. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009; 35:Suppl 1. 302–312.
Article15. England MD, Cavarocchi NC, O'brien JF, Solis E, Pluth JR, Orszulak TA, et al. Influence of antioxidants (mannitol and allopurinol) on oxygen free radical generation during and after cardiopulmonary bypass. Circulation. 1986; 74:III134–III137.16. Weinbroum AA. Mannitol prevents acute lung injury after pancreas ischemia-reperfusion: a dose-response, ex vivo study. Lung. 2009; 187:215–224.
Article17. Liu JH, Wann H, Chen MM, Pan WH, Chen YC, Liu CM, et al. Baicalein significantly protects human retinal pigment epithelium cells against H2O2-induced oxidative stress by scavenging reactive oxygen species and downregulating the expression of matrix metalloproteinase-9 and vascular endothelial growth factor. J Ocul Pharmacol Ther. 2010; 26:421–429.
Article18. Khoury W, Namnesnikov M, Fedorov D, Abu-Gazala S, Weinbroum AA. Mannitol attenuates kidney damage induced by xanthine oxidase-associated pancreas ischemia-reperfusion. J Surg Res. 2010; 160:163–168.
Article19. Belda JI, Artola A, Garcia-Manzanares MD, Ferrer C, Haroun HE, Hassanein A, et al. Hyaluronic acid combined with mannitol to improve protection against free-radical endothelial damage: experimental model. J Cataract Refract Surg. 2005; 31:1213–1218.
Article20. Baspeyras M, Rouvrais C, Liegard L, Delalleau A, Letellier S, Bacle I, et al. Clinical and biometrological efficacy of a hyaluronic acid-based mesotherapy product: a randomised controlled study. Arch Dermatol Res. 2013; 305:673–682.
Article21. Taieb M, Gay C, Sebban S, Secnazi P. Hyaluronic acid plus mannitol treatment for improved skin hydration and elasticity. J Cosmet Dermatol. 2012; 11:87–92.
Article22. Lemperle G, Holmes RE, Cohen SR, Lemperle SM. A classification of facial wrinkles. Plast Reconstr Surg. 2001; 108:1735–1750. discussion 1751-1752.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the efficacy and safety between a new monophasic hyaluronic acid filler and a biphasic hyaluronic acid filler in correcting facial wrinkles
- Efficacy and Safety of Porcine Collagen Filler for Nasolabial Fold Correction in Asians: A Prospective Multicenter, 12 Months Follow-up Study
- Recurrent delayed hypersensitivity reaction to a hyaluronic acid soft-tissue filler following COVID-19 vaccination: a case report
- Delayed skin discoloration spreading in the direction of gravity after injection of hyaluronic acid dermal fillers in both nasolabial folds
- Immediate improvement of alar drooping and nostril shape by hyaluronic acid filler injection into the perialar regions: a case study