J Korean Med Sci.
2014 Nov;29(Suppl 3):S183-S192. 10.3346/jkms.2014.29.S3.S183.
In Vivo Effects of Adipose-Derived Stem Cells in Inducing Neuronal Regeneration in Sprague-Dawley Rats Undergoing Nerve Defect Bridged with Polycaprolactone Nanotubes
- Affiliations
-
- 1Department of Plastic and Reconstructive Surgery, The Catholic University of Korea, Seoul, Korea. joony@catholic.ac.kr
- 2Department of Orthopedic Surgery and Rare Diseases Institute, Korea University, Seoul, Korea.
- KMID: 2151412
- DOI: http://doi.org/10.3346/jkms.2014.29.S3.S183
Abstract
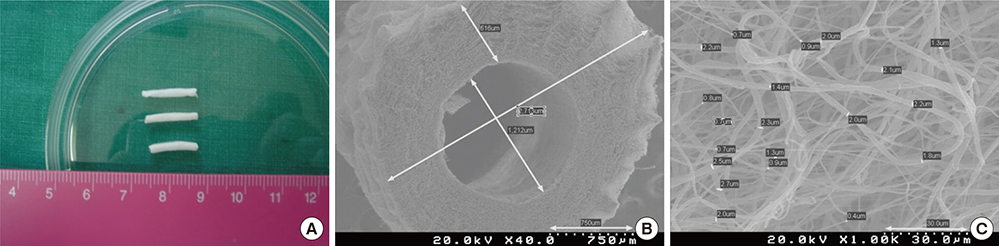
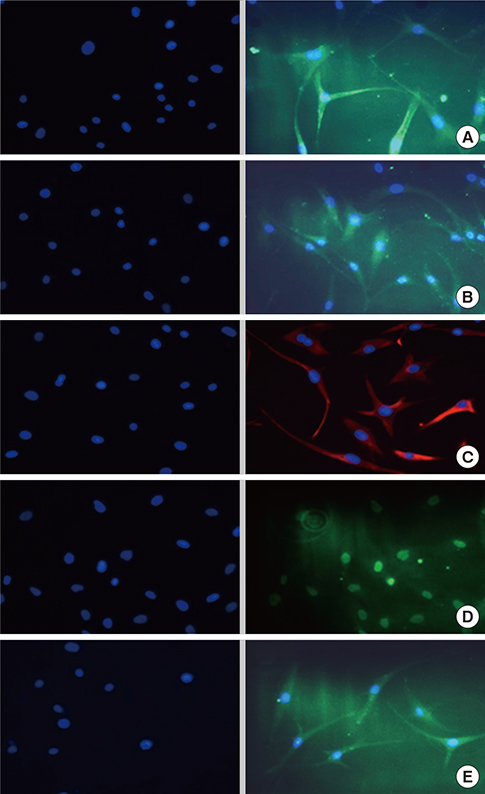
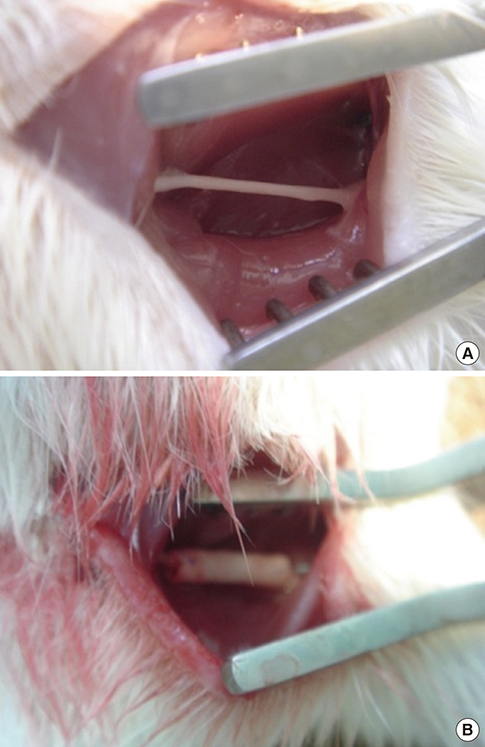
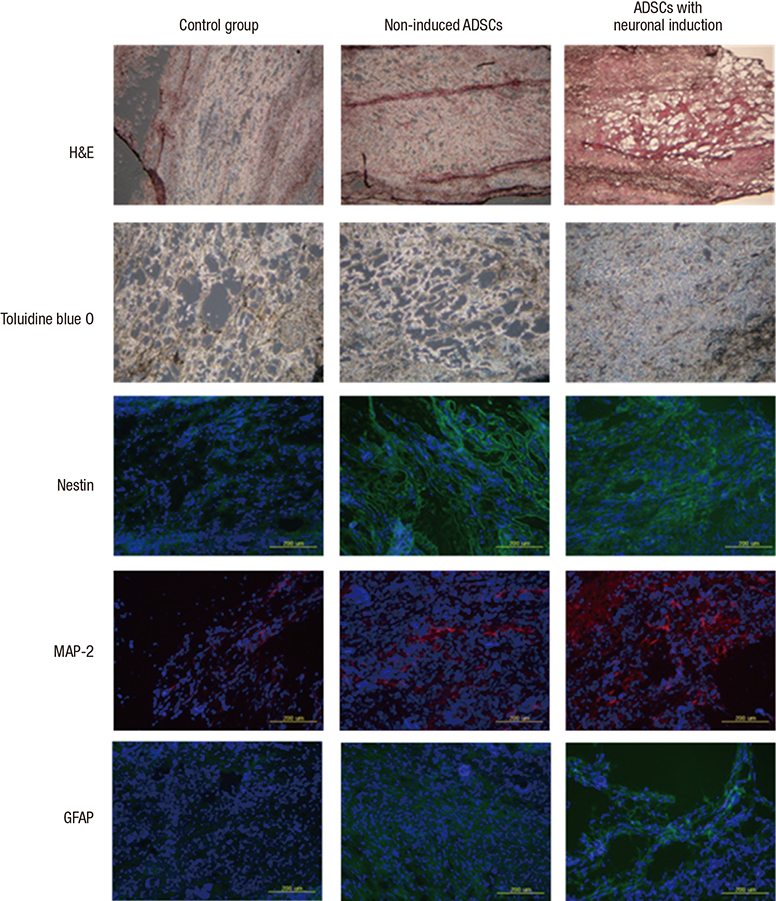
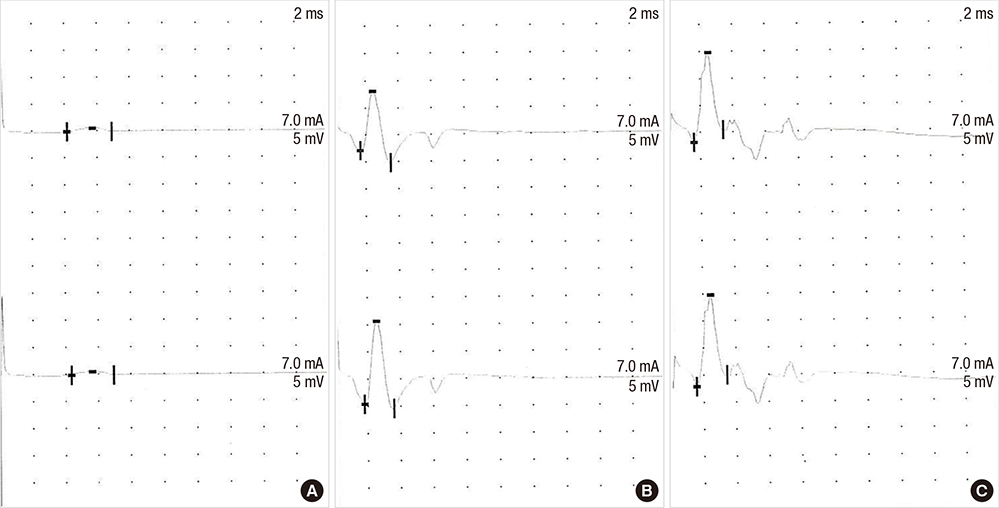
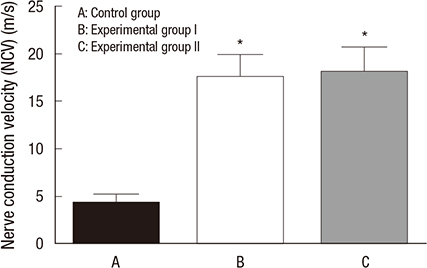
- There have been many attempts for regeneration of peripheral nerve injury. In this study, we examined the in vivo effects of non-differentiated and neuronal differentiated adipose-derived stem cells (ADSCs) in inducing the neuronal regeneration in the Sprague-Dawley (SD) rats undergoing nerve defect bridged with the PCL nanotubes. Then, we performed immunohistochemical and histopathologic examinations, as well as the electromyography, in three groups: the control group (14 sciatic nerves transplanted with the PCL nanotube scaffold), the experimental group I (14 sciatic nerves with the non-differentiated ADSCs at a density of 7x105 cells/0.1 mL) and the experimental group II (14 sciatic nerves with the neuronal differentiated ADSCs at 7x105 cells/0.1 mL). Six weeks postoperatively, the degree of the neuronal induction and that of immunoreactivity to nestin, MAP-2 and GFAP was significantly higher in the experimental group I and II as compared with the control group. In addition, the nerve conduction velocity (NCV) was significantly higher in the experimental group I and II as compared with the control group (P=0.021 and P=0.020, respectively). On the other hand, there was no significant difference in the NCV between the two experimental groups (P>0.05). Thus, our results will contribute to treating patients with peripheral nerve defects using PCL nanotubes with ADSCs.
Keyword
MeSH Terms
-
Adipose Tissue/cytology
Animals
Cell Differentiation
Electromyography
Male
Nanotubes
*Nerve Regeneration
Nerve Tissue Proteins/immunology
Nestin/immunology
Neural Conduction/physiology
Peripheral Nerve Injuries/*surgery
Phosphoprotein Phosphatases/immunology
Polyesters/*therapeutic use
Rats
Rats, Sprague-Dawley
Sciatic Nerve/injuries/surgery
Stem Cell Transplantation/*methods
Stem Cells/*cytology
Tissue Engineering/methods
Nerve Tissue Proteins
Nestin
Polyesters
Phosphoprotein Phosphatases
Figure
Reference
-
1. Martínez de Albornoz P, Delgado PJ, Forriol F, Maffulli N. Non-surgical therapies for peripheral nerve injury. Br Med Bull. 2011; 100:73–100.2. Lin MY, Manzano G, Gupta R. Nerve allografts and conduits in peripheral nerve repair. Hand Clin. 2013; 29:331–348.3. Hallgren A, Björkman A, Chemnitz A, Dahlin LB. Subjective outcome related to donor site morbidity after sural nerve graft harvesting: a survey in 41 patients. BMC Surg. 2013; 13:39.4. Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol. 2007; 207:267–274.5. Papalia I, Raimondo S, Ronchi G, Magaudda L, Giacobini-Robecchi MG, Geuna S. Repairing nerve gaps by vein conduits filled with lipoaspirate-derived entire adipose tissue hinders nerve regeneration. Ann Anat. 2013; 195:225–230.6. Zhang Y, Luo H, Zhang Z, Lu Y, Huang X, Yang L, Xu J, Yang W, Fan X, Du B, et al. A nerve graft constructed with xenogeneic acellular nerve matrix and autologous adipose-derived mesenchymal stem cells. Biomaterials. 2010; 31:5312–5324.7. Wang Y, Zhao Z, Ren Z, Zhao B, Zhang L, Chen J, Xu W, Lu S, Zhao Q, Peng J. Recellularized nerve allografts with differentiated mesenchymal stem cells promote peripheral nerve regeneration. Neurosci Lett. 2012; 514:96–101.8. Lim EH, Sardinha JP, Myers S. Nanotechnology biomimetic cartilage regenerative scaffolds. Arch Plast Surg. 2014; 41:231–240.9. Lietz M, Dreesmann L, Hoss M, Oberhoffner S, Schlosshauer B. Neuro tissue engineering of glial nerve guides and the impact of different cell types. Biomaterials. 2006; 27:1425–1436.10. Alluin O, Wittmann C, Marqueste T, Chabas JF, Garcia S, Lavaut MN, Guinard D, Feron F, Decherchi P. Functional recovery after peripheral nerve injury and implantation of a collagen guide. Biomaterials. 2009; 30:363–373.11. Kim JG, Rhee SC, Cho PD, Kim DJ, Lee SH. Absorbable plate as a perpendicular strut for acute saddle nose deformities. Arch Plast Surg. 2012; 39:113–117.12. Chia HL, Wong M, Tan BK. Nipple reconstruction with rolled dermal graft support. Arch Plast Surg. 2014; 41:158–162.13. Salibian AA, Widgerow AD, Abrouk M, Evans GR. Stem cells in plastic surgery: a review of current clinical and translational applications. Arch Plast Surg. 2013; 40:666–675.14. Lee JH, Lee KH, Kim MH, Kim JP, Lee SJ, Yoon J. Possibility of undifferentiated human thigh adipose stem cells differentiating into functional hepatocytes. Arch Plast Surg. 2012; 39:593–599.15. Chen CJ, Ou YC, Liao SL, Chen WY, Chen SY, Wu CW, Wang CC, Wang WY, Huang YS, Hsu SH. Transplantation of bone marrow stromal cells for peripheral nerve repair. Exp Neurol. 2007; 204:443–453.16. Yang JD, Choi DS, Cho YK, Kim TK, Lee JW, Choi KY, Chung HY, Cho BC, Byun JS. Effect of amniotic fluid stem cells and amniotic fluid cells on the wound healing process in a white rat model. Arch Plast Surg. 2013; 40:496–504.17. Choi J, Minn KW, Chang H. The efficacy and safety of platelet-rich plasma and adipose-derived stem cells: an update. Arch Plast Surg. 2012; 39:585–592.18. Radtke C, Schmitz B, Spies M, Kocsis JD, Vogt PM. Peripheral glial cell differentiation from neurospheres derived from adipose mesenchymal stem cells. Int J Dev Neurosci. 2009; 27:817–823.19. Johnson TS, O'Neill AC, Motarjem PM, Nazzal J, Randolph M, Winograd JM. Tumor formation following murine neural precursor cell transplantation in a rat peripheral nerve injury model. J Reconstr Microsurg. 2008; 24:545–550.20. Swijnenburg RJ, Schrepfer S, Cao F, Pearl JI, Xie X, Connolly AJ, Robbins RC, Wu JC. In vivo imaging of embryonic stem cells reveals patterns of survival and immune rejection following transplantation. Stem Cells Dev. 2008; 17:1023–1029.21. Dalton PD, Lleixà Calvet J, Mourran A, Klee D, Möller M. Melt electrospinning of poly-(ethylene glycol-block-epsilon-caprolactone). Biotechnol J. 2006; 1:998–1006.22. Sun XH, Che YQ, Tong XJ, Zhang LX, Feng Y, Xu AH, Tong L, Jia H, Zhang X. Improving nerve regeneration of acellular nerve allografts seeded with SCs bridging the sciatic nerve defects of rat. Cell Mol Neurobiol. 2009; 29:347–353.23. Bozkurt A, Deumens R, Beckmann C, Olde Damink L, Schügner F, Heschel I, Sellhaus B, Weis J, Jahnen-Dechent W, Brook GA, et al. In vitro cell alignment obtained with a Schwann cell enriched microstructured nerve guide with longitudinal guidance channels. Biomaterials. 2009; 30:169–179.24. Chia HL, Yeow VK. Repair of inferior sternal cleft using bilateral sternal bar turnover flaps in a patient with pentalogy of cantrell. Arch Plast Surg. 2014; 41:77–80.25. Takayanagi S. Augmentation mammaplasty using implants: a review. Arch Plast Surg. 2012; 39:448–451.26. Yannas IV, Hill BJ. Selection of biomaterials for peripheral nerve regeneration using data from the nerve chamber model. Biomaterials. 2004; 25:1593–1600.27. Rodríguez FJ, Verdú E, Ceballos D, Navarro X. Nerve guides seeded with autologous schwann cells improve nerve regeneration. Exp Neurol. 2000; 161:571–584.28. Sun M, Downes S. Physicochemical characterisation of novel ultra-thin biodegradable scaffolds for peripheral nerve repair. J Mater Sci Mater Med. 2009; 20:1181–1192.29. Nguyen HT, Sapp S, Wei C, Chow JK, Nguyen A, Coursen J, Luebben S, Chang E, Ross R, Schmidt CE. Electric field stimulation through a biodegradable polypyrrole-co-polycaprolactone substrate enhances neural cell growth. J Biomed Mater Res A. 2014; 102:2554–2564.30. Yan H, Zhang F, Chen MB, Lineaweaver WC. Chapter 10: Conduit luminal additives for peripheral nerve repair. Int Rev Neurobiol. 2009; 87:199–225.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Adipose Derived Stem Cells on Neurogenic Differentiation and Induction of Nerve Regeneration
- Bone Regeneration from Adipose Stem Cells
- T Lymphocyte Subsets and Cytokines in Rats Transplanted with Adipose-Derived Mesenchymal Stem Cells and Acellular Nerve for Repairing the Nerve Defects
- Adipose Tissue - Adequate, Accessible Regenerative Material
- Novel Calcium Phosphate Glass for Hard-Tissue Regeneration