Immune Netw.
2014 Aug;14(4):207-218. 10.4110/in.2014.14.4.207.
Enhancing T Cell Immune Responses by B Cell-based Therapeutic Vaccine Against Chronic Virus Infection
- Affiliations
-
- 1System Immunology Laboratory, Department of Biochemistry, College of Life Science and Biotechnology, Yonsei University, Seoul 120-749, Korea. sjha@yonsei.ac.kr
- 2Cell Therapy Team, Mogam Biotechnology Institute, Yongin 446-799, Korea.
- 3College of Pharmacy, Seoul National University, Seoul 110-799, Korea.
- KMID: 2150809
- DOI: http://doi.org/10.4110/in.2014.14.4.207
Abstract
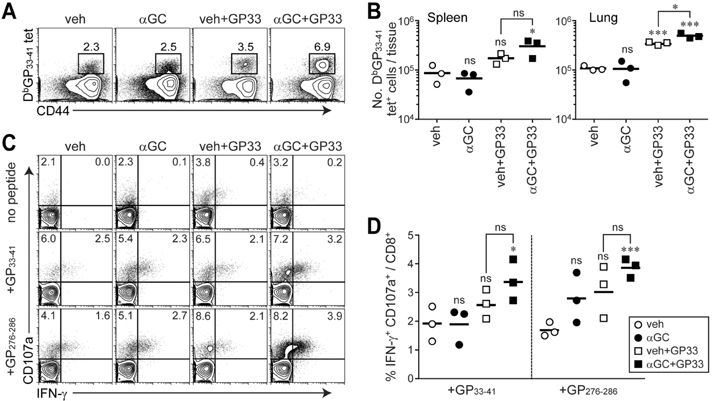
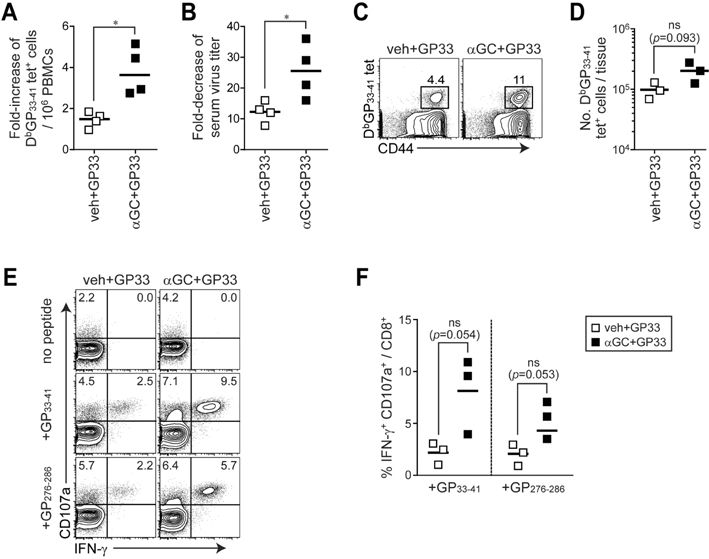
- Chronic virus infection leads to the functional impairment of dendritic cells (DCs) as well as T cells, limiting the clinical usefulness of DC-based therapeutic vaccine against chronic virus infection. Meanwhile, B cells have been known to maintain the ability to differentiate plasma cells producing antibodies even during chronic virus infection. Previously, alpha-galactosylceramide (alphaGC) and cognate peptide-loaded B cells were comparable to DCs in priming peptide-specific CD8+ T cells as antigen presenting cells (APCs). Here, we investigated whether B cells activated by alphaGC can improve virus-specific T cell immune responses instead of DCs during chronic virus infection. We found that comparable to B cells isolated from naive mice, chronic B cells isolated from chronically infected mice with lymphocytic choriomeningitis virus (LCMV) clone 13 (CL13) after alphaGC-loading could activate CD1d-restricted invariant natural killer T (iNKT) cells to produce effector cytokines and upregulate co-stimulatory molecules in both naive and chronically infected mice. Similar to naive B cells, chronic B cells efficiently primed LCMV glycoprotein (GP) 33-41-specific P14 CD8+ T cells in vivo, thereby allowing the proliferation of functional CD8+ T cells. Importantly, when alphaGC and cognate epitope-loaded chronic B cells were transferred into chronically infected mice, the mice showed a significant increase in the population of epitope-specific CD8+ T cells and the accelerated control of viremia. Therefore, our studies demonstrate that reciprocal activation between alphaGC-loaded chronic B cells and iNKT cells can strengthen virus-specific T cell immune responses, providing an effective regimen of autologous B cell-based therapeutic vaccine to treat chronic virus infection.
Keyword
MeSH Terms
Figure
Reference
-
1. Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003; 77:4911–4927.
Article2. Ha SJ, West EE, Araki K, Smith KA, Ahmed R. Manipulating both the inhibitory and stimulatory immune system towards the success of therapeutic vaccination against chronic viral infections. Immunol Rev. 2008; 223:317–333.
Article3. Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009; 138:30–50.
Article4. Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998; 188:2205–2213.
Article5. Jin HT, Jeong YH, Park HJ, Ha SJ. Mechanism of T cell exhaustion in a chronic environment. BMB Rep. 2011; 44:217–231.
Article6. Ng CT, Snell LM, Brooks DG, Oldstone MB. Networking at the level of host immunity: immune cell interactions during persistent viral infections. Cell Host Microbe. 2013; 13:652–664.
Article7. Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med. 2011; 208:987–999.
Article8. Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004; 10:909–915.
Article9. Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic Cell Immunotherapy: Mapping The Way. Nat Med. 2004; 10:475–480.
Article10. Janikashvili N, Larmonier N, Katsanis E. Personalized dendritic cell-based tumor immunotherapy. Immunotherapy. 2010; 2:57–68.
Article11. Schultze JL, Grabbe S, von Bergwelt-Baildon MS. DCs and CD40-activated B cells: current and future avenues to cellular cancer immunotherapy. Trends Immunol. 2004; 25:659–664.
Article12. Eynon EE, Parker DC. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992; 175:131–138.
Article13. Fuchs EJ, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992; 258:1156–1159.
Article14. Schultze JL, Michalak S, Seamon MJ, Dranoff G, Jung K, Daley J, Delgado JC, Gribben JG, Nadler LM. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest. 1997; 100:2757–2765.
Article15. Coughlin CM, Vance BA, Grupp SA, Vonderheide RH. RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood. 2004; 103:2046–2054.
Article16. Lapointe R, Bellemare-Pelletier A, Housseau F, Thibodeau J, Hwu P. CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res. 2003; 63:2836–2843.17. Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007; 25:297–336.
Article18. Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010; 11:197–206.
Article19. Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005; 23:877–900.
Article20. Park SH, Bendelac A. CD1-restricted T-cell responses and microbial infection. Nature. 2000; 406:788–792.
Article21. Chung Y, Kim BS, Kim YJ, Ko HJ, Ko SY, Kim DH, Kang CY. CD1d-restricted T cells license B cells to generate long-lasting cytotoxic antitumor immunity in vivo. Cancer Res. 2006; 66:6843–6850.
Article22. Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004; 114:1379–1388.
Article23. Kim YJ, Ko HJ, Kim YS, Kim DH, Kang S, Kim JM, Chung Y, Kang CY. alpha-Galactosylceramide-loaded, antigen-expressing B cells prime a wide spectrum of antitumor immunity. Int J Cancer. 2008; 122:2774–2783.
Article24. Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, Schmidt R, Harris AL, Old L, Cerundolo V. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003; 171:5140–5147.
Article25. Stober D, Jomantaite I, Schirmbeck R, Reimann J. NKT cells provide help for dendritic cell-dependent priming of MHC class I-restricted CD8+ T cells in vivo. J Immunol. 2003; 170:2540–2548.
Article26. Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009; 9:28–38.
Article27. Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, Freeman GJ, Ahmed R. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008; 205:543–555.
Article28. Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006; 439:682–687.
Article29. King CC, de Fries R, Kolhekar SR, Ahmed R. In vivo selection of lymphocyte-tropic and macrophage-tropic variants of lymphocytic choriomeningitis virus during persistent infection. J Virol. 1990; 64:5611–5616.
Article30. Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009; 324:1569–1572.
Article31. Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009; 324:1576–1580.
Article32. Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009; 324:1572–1576.
Article33. Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010; 207:353–363.
Article34. Zotos D, Coquet JM, Zhang Y, Light A, D'Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, Nutt SL, Tarlinton DM. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010; 207:365–378.
Article35. Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002; 2:116–126.
Article36. Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007; 27:670–684.
Article37. Wiesner M, Zentz C, Mayr C, Wimmer R, Hammerschmidt W, Zeidler R, Moosmann A. Conditional immortalization of human B cells by CD40 ligation. PLoS One. 2008; 3:e1464.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Porcine reproductive and respiratory syndrome virus vaccine does not fit in classical vaccinology
- Baculovirus-based Vaccine Displaying Respiratory Syncytial Virus Glycoprotein Induces Protective Immunity against RSV Infection without Vaccine-Enhanced Disease
- Enhancement of DNA Vaccine-induced Immune Responses by Influenza Virus NP Gene
- Vaccine Strategy That Enhances the Protective Efficacy of Systemic Immunization by Establishing LungResident Memory CD8 T Cells Against Influenza Infection
- The Role of CD4 T Cell Help in CD8 T Cell Differentiation and Function During Chronic Infection and Cancer