Immune Netw.
2013 Feb;13(1):16-24. 10.4110/in.2013.13.1.16.
Functional Characteristics of C-terminal Lysine to Cysteine Mutant Form of CTLA-4Ig
- Affiliations
-
- 1Department of Microbiology and Immunology, Seoul National University College of Medicine, Seoul 110-799, Korea. chgpark@snu.ac.kr
- 2Cancer Research Institute, Seoul National University College of Medicine, Seoul 110-799, Korea.
- 3Xenotransplantation Research Center, Seoul National University College of Medicine, Seoul 110-799, Korea.
- KMID: 2150766
- DOI: http://doi.org/10.4110/in.2013.13.1.16
Abstract
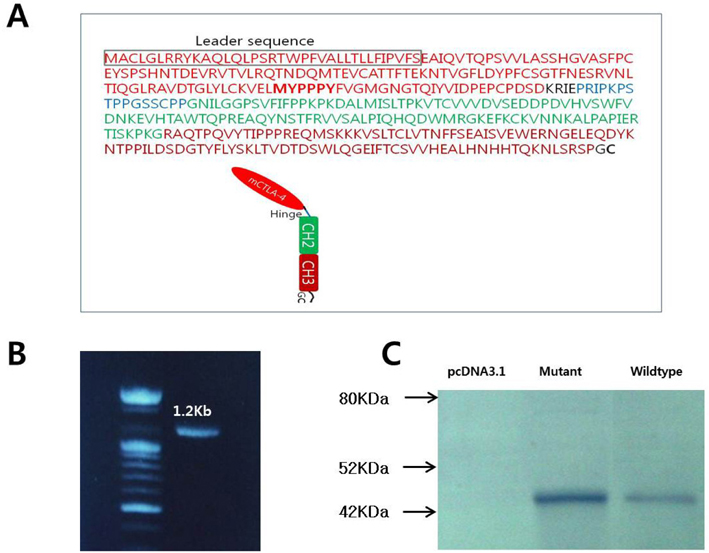
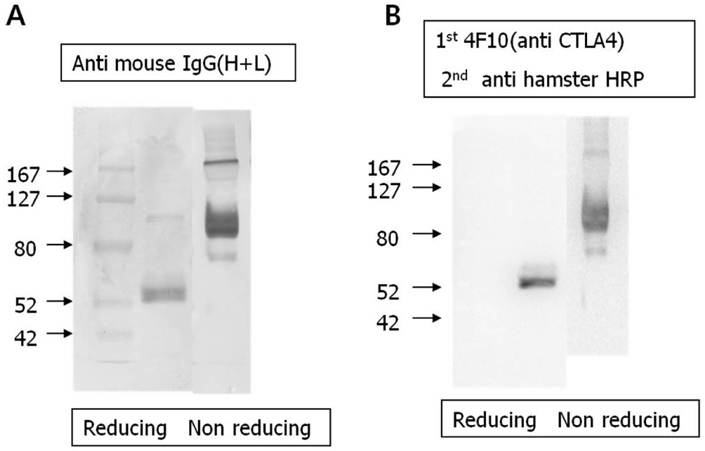
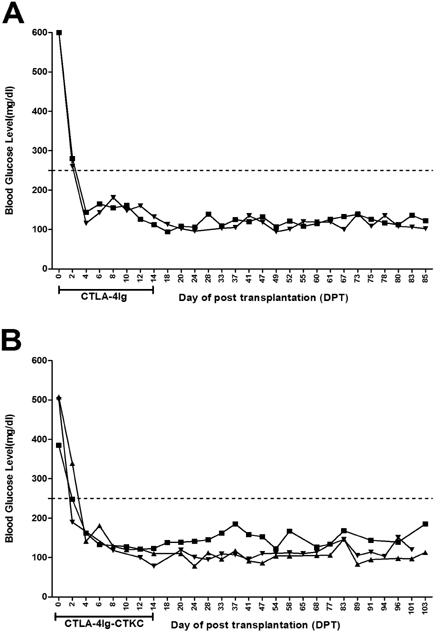
- CTLA-4Ig is regarded as an inhibitory agent of the T cell proliferation via blocking the costimulatory signal which is essential for full T cell activation. To improve applicability, we developed the CTLA-4Ig-CTKC in which the c-terminal lysine had been replaced by cysteine through single amino acid change. The single amino acid mutation of c-terminus of CTLA-4Ig was performed by PCR and was checked by in vitro transcription and translation. DNA construct of mutant form was transfected to Chinese hamster ovary (CHO) cells by electroporation. The purified proteins were confirmed by Western blot and B7-1 binding assay for their binding ability. The suppressive capacity of CTLA-4Ig-CTKC was evaluated by the mixed lymphocyte reaction (MLR) and in the allogeneic pancreatic islet transplantation model. CTLA-4Ig-CTKC maintained binding ability to B7-1 molecule and effectively inhibits T cell proliferation in MLR. In the murine allogeneic pancreatic islet transplantation, short-term treatment of CTLA-4Ig-CTKC prolonged the graft survival over 100 days. CTLA-4Ig-CTKC effectively inhibits immune response both in MLR and in allogeneic islet transplantation model, indicating that single amino acid mutation does not affect the inhibitory function of CTLA-4Ig. CTLA-4Ig-CTKC can be used in vehicle-mediated drug delivery system such as liposome conjugation.
Keyword
MeSH Terms
-
Animals
Blotting, Western
Cell Proliferation
Cricetinae
Cricetulus
Cysteine
DNA
Drug Delivery Systems
Electroporation
Female
Graft Survival
Islets of Langerhans
Islets of Langerhans Transplantation
Liposomes
Lymphocyte Culture Test, Mixed
Lysine
Ovary
Polymerase Chain Reaction
Proteins
Transplants
Cysteine
DNA
Liposomes
Lysine
Proteins
Figure
Reference
-
1. Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996. 14:233–258.
Article2. Lafferty KJ, Prowse SJ, Simeonovic CJ, Warren HS. Immunobiology of tissue transplantation: a return to the passenger leukocyte concept. Annu Rev Immunol. 1983. 1:143–173.
Article3. June CH, Bluestone JA, Nadler LM, Thompson CB. The B7 and CD28 receptor families. Immunol Today. 1994. 15:321–331.
Article4. Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001. 19:225–252.
Article5. Greenfield EA, Nguyen KA, Kuchroo VK. CD28/B7 costimulation: a review. Crit Rev Immunol. 1998. 18:389–418.
Article6. Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994. 1:405–413.
Article7. Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006. 24:65–97.
Article8. Lenschow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, Linsley PS, Bluestone JA. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992. 257:789–792.
Article9. Turka LA, Linsley PS, Lin H, Brady W, Leiden JM, Wei RQ, Gibson ML, Zheng XG, Myrdal S, Gordon D, et al. T-cell activation by the CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc Natl Acad Sci U S A. 1992. 89:11102–11105.
Article10. Li W, Lu L, Wang Z, Wang L, Fung JJ, Thomson AW, Qian S. Costimulation blockade promotes the apoptotic death of graft-infiltrating T cells and prolongs survival of hepatic allografts from FLT3L-treated donors. Transplantation. 2001. 72:1423–1432.
Article11. Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobert E, Anderson D, Cowan S, Price K, Naemura J, Emswiler J, Greene J, Turk LA, Bajorath J, Townsend R, Hagerty D, Linsley PS, Peach RJ. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005. 5:443–453.
Article12. Park CG, Thiex NW, Lee KM, Szot GL, Bluestone JA, Lee KD. Targeting and blocking B7 costimulatory molecules on antigen-presenting cells using CTLA4Ig-conjugated liposomes: in vitro characterization and in vivo factors affecting biodistribution. Pharm Res. 2003. 20:1239–1248.13. Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967. 16:35–39.
Article14. Abrams JR, Lebwohl MG, Guzzo CA, Jegasothy BV, Goldfarb MT, Goffe BS, Menter A, Lowe NJ, Krueger G, Brown MJ, Weiner RS, Birkhofer MJ, Warner GL, Berry KK, Linsley PS, Krueger JG, Ochs HD, Kelley SL, Kang S. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999. 103:1243–1252.
Article15. Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, Birbara C, Box J, Natarajan K, Nuamah I, Li T, Aranda R, Hagerty DT, Dougados M. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005. 353:1114–1123.
Article16. Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S, Russell A, Dougados M, Emery P, Nuamah IF, Williams GR, Becker JC, Hagerty DT, Moreland LW. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003. 349:1907–1915.
Article17. Kaplan B. Belatacept: the promises and challenges of belatacept and costimulatory blockade. Am J Transplant. 2010. 10:441–442.
Article18. Larsen CP, Grinyó J, Medina-Pestana J, Vanrenterghem Y, Vincenti F, Breshahan B, Campistol JM, Florman S, Rial Mdel C, Kamar N, Block A, Di Russo G, Lin CS, Garg P, Charpentier B. Belatacept-based regimens versus a cyclosporine A-based regimen in kidney transplant recipients: 2-year results from the BENEFIT and BENEFIT-EXT studies. Transplantation. 2010. 90:1528–1535.
Article19. Vincenti F, Blancho G, Durrbach A, Friend P, Grinyo J, Halloran PF, Klempnauer J, Lang P, Larsen CP, Mühlbacher F, Nashan B, Soulillou JP, Vanrenterghem Y, Wekerle T, Agarwal M, Gujrathi S, Shen J, Shi R, Townsend R, Charpentier B. Five-year safety and efficacy of belatacept in renal transplantation. J Am Soc Nephrol. 2010. 21:1587–1596.
Article20. Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, Massari P, Mondragon-Ramirez GA, Agarwal M, Di Russo G, Lin CS, Garg P, Larsen CP. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant. 2010. 10:535–546.
Article21. Durrbach A, Pestana JM, Pearson T, Vincenti F, Garcia VD, Campistol J, Rial Mdel C, Florman S, Block A, Di Russo G, Xing J, Garg P, Grinyó J. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study). Am J Transplant. 2010. 10:547–557.
Article22. Alegre ML, Tso JY, Sattar HA, Smith J, Desalle F, Cole M, Bluestone JA. An anti-murine CD3 monoclonal antibody with a low affinity for Fc gamma receptors suppresses transplantation responses while minimizing acute toxicity and immunogenicity. J Immunol. 1995. 155:1544–1555.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of N-terminal Hydrophilic Amino Acids in Molecular Translocation of CTLA-4 to Cell Surface
- Characterization of cytoplasmic Form of Human CTLA - 4 Molecule
- N-acetyl-L-cysteine and cysteine increase intracellular calcium concentration in human neutrophils
- Cysteine-Added Mutants of Turnip Yellow Mosaic Virus
- Evaluation of expression patterns of feline CD28 and CTLA-4 in feline immunodeficiency virus (FIV)-infected and FIV antigen-induced PBMC