Immune Netw.
2011 Dec;11(6):368-375. 10.4110/in.2011.11.6.368.
Blockade of Vascular Endothelial Growth Factor (VEGF) Aggravates the Severity of Acute Graft-versus-host Disease (GVHD) after Experimental Allogeneic Hematopoietic Stem Cell Transplantation (allo-HSCT)
- Affiliations
-
- 1Department of Pediatrics, The Catholic University of Korea, Seoul 137-701, Korea.
- 2Department of Internal Medicine, The Catholic University of Korea, Seoul 137-701, Korea. ckmin@catholic.ac.kr
- 3Department of Hospital Pathology, The Catholic University of Korea, Seoul 137-701, Korea.
- KMID: 2150722
- DOI: http://doi.org/10.4110/in.2011.11.6.368
Abstract
- BACKGROUND
Recent clinical observation reported that there was a significant correlation between change in circulating vascular endothelial growth factor (VEGF) levels and the occurrence of severe acute graft-versus-host disease (GVHD) following allogeneic hematopoietic stem cell transplantation (allo-HSCT), but the action mechanisms of VEGF in GVHD have not been demonstrated.
METHODS
This study investigated whether or not blockade of VEGF has an effect on acute GVHD in a lethally irradiated murine allo-HSCT model of B6 (H-2b)-->B6D2F1 (H-2b/d). Syngeneic or allogeneic recipient mice were injected subcutaneously with anti-VEGF peptides, dRK6 (50 microg/dose) or control diluent every other day for 2 weeks (total 7 doses).
RESULTS
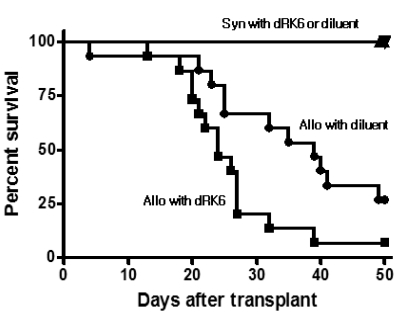
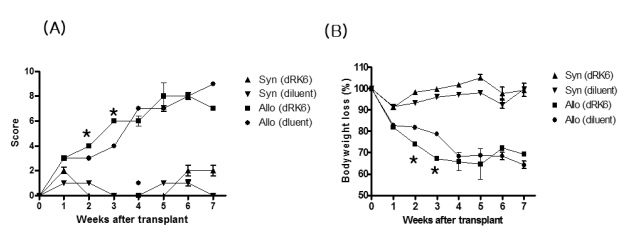
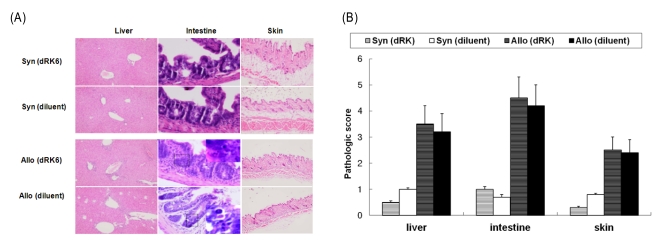
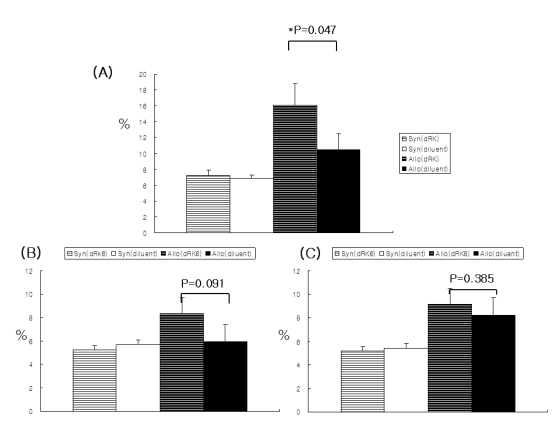
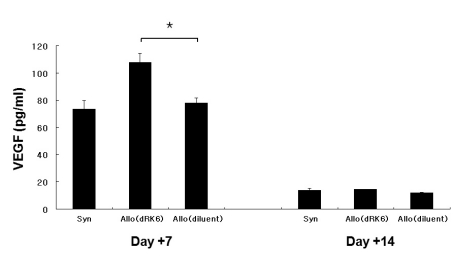
Administration of the dRK6 peptide after allo-HSCT significantly reduced survival with greaterclinical GVHD scores and body weight loss. Allogeneic recipients injected with the dRK6 peptide exhibited significantly increased circulating levels of VEGF and expansion of donor CD3+ T cells on day +7 compared to control treated animals. The donor CD4+ and CD8+ T-cell subsets have differential expansion caused by the dRK6 injection. The circulating VEGF levels were reduced on day +14 regardless of blockade of VEGF.
CONCLUSION
Together these findings demonstrate that the allo-reactive responses after allo-HSCT are exaggerated by the blockade of VEGF. VEGF seems to be consumed during the progression of acute GVHD in this murine allo-HSCT model.
Keyword
MeSH Terms
Figure
Reference
-
1. Devine SM, Adkins DR, Khoury H, Brown RA, Vij R, Blum W, DiPersio JF. Recent advances in allogeneic hematopoietic stem-cell transplantation. J Lab Clin Med. 2003; 141:7–32. PMID: 12518165.
Article2. Wingard JR. Opportunistic infections after blood and marrow transplantation. Transpl Infect Dis. 1999; 1:3–20. PMID: 11428967.
Article3. Takatsuka H, Takemoto Y, Yamada S, Wada H, Tamura S, Fujimori Y, Okamoto T, Suehiro A, Kanamaru A, Kakishita E. Complications after bone marrow transplantation are manifestations of systemic inflammatory response syndrome. Bone Marrow Transplant. 2000; 26:419–426. PMID: 10982289.
Article4. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992; 20:864–874. PMID: 1597042.5. Chen X, Christou NV. Relative contribution of endothelial cell and polymorphonuclear neutrophil activation in their interactions in systemic inflammatory response syndrome. Arch Surg. 1996; 131:1148–1153. PMID: 8911254.
Article6. Klagsbrun M, D'Amore PA. Regulators of angiogenesis. Annu Rev Physiol. 1991; 53:217–239. PMID: 1710435.
Article7. Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, micro-vascular hyperpermeability, and angiogenesis. Am J Pathol. 1995; 146:1029–1039. PMID: 7538264.8. Fava RA, Olsen NJ, Spencer-Green G, Yeo KT, Yeo TK, Berse B, Jackman RW, Senger DR, Dvorak HF, Brown LF. Vascular permeability factor/endothelial growth factor (VPF/VEGF): accumulation and expression in human synovial fluids and rheumatoid synovial tissue. J Exp Med. 1994; 180:341–346. PMID: 8006592.
Article9. Selvaraj SK, Giri RK, Perelman N, Johnson C, Malik P, Kalra VK. Mechanism of monocyte activation and expression of proinflammatory cytochemokines by placenta growth factor. Blood. 2003; 102:1515–1524. PMID: 12689930.
Article10. Iyer S, Leonidas DD, Swaminathan GJ, Maglione D, Battisti M, Tucci M, Persico MG, Acharya KR. The crystal structure of human placenta growth factor-1 (PlGF-1), an angiogenic protein, at 2.0 A resolution. J Biol Chem. 2001; 276:12153–12161. PMID: 11069911.11. Lunn RA, Sumar N, Bansal AS, Treleaven J. Cytokine profiles in stem cell transplantation: possible use as a predictor of graft-versus-host disease. Hematology. 2005; 10:107–114. PMID: 16019456.
Article12. Min CK, Kim SY, Lee MJ, Eom KS, Kim YJ, Kim HJ, Lee S, Cho SG, Kim DW, Lee JW, Min WS, Kim CC, Cho CS. Vascular endothelial growth factor (VEGF) is associated with reduced severity of acute graft-versus-host disease and nonrelapse mortality after allogeneic stem cell transplantation. Bone Marrow Transplant. 2006; 38:149–156. PMID: 16751784.
Article13. Bae DG, Gho YS, Yoon WH, Chae CB. Arginine-rich anti-vascular endothelial growth factor peptides inhibit tumor growth and metastasis by blocking angiogenesis. J Biol Chem. 2000; 275:13588–13596. PMID: 10788475.
Article14. Min CK, Maeda Y, Lowler K, Liu C, Clouthier S, Lofthus D, Weisiger E, Ferrara JL, Reddy P. Paradoxical effects of inter-leukin-18 on the severity of acute graft-versus-host disease mediated by CD4+ and CD8+ T-cell subsets after experimental allogeneic bone marrow transplantation. Blood. 2004; 104:3393–3399. PMID: 15280194.
Article15. Asai O, Longo DL, Tian ZG, Hornung RL, Taub DD, Ruscetti FW, Murphy WJ. Suppression of graft-versus-host disease and amplification of graft-versus-tumor effects by activated natural killer cells after allogeneic bone marrow transplantation. J Clin Invest. 1998; 101:1835–1842. PMID: 9576746.
Article16. Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte J Jr, Crawford JM, Ferrara JL. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996; 88:3230–3239. PMID: 8963063.
Article17. Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997; 90:3204–3213. PMID: 9376604.
Article18. Yoo SA, Bae DG, Ryoo JW, Kim HR, Park GS, Cho CS, Chae CB, Kim WU. Arginine-rich anti-vascular endothelial growth factor (anti-VEGF) hexapeptide inhibits collagen-induced arthritis and VEGF-stimulated productions of TNF-alpha and IL-6 by human monocytes. J Immunol. 2005; 174:5846–5855. PMID: 15843589.19. Reddy P, Teshima T, Kukuruga M, Ordemann R, Liu C, Lowler K, Ferrara JL. Interleukin-18 regulates acute graft-versus-host disease by enhancing Fas-mediated donor T cell apoptosis. J Exp Med. 2001; 194:1433–1440. PMID: 11714750.
Article20. Tsuchihashi S, Ke B, Kaldas F, Flynn E, Busuttil RW, Briscoe DM, Kupiec-Weglinski JW. Vascular endothelial growth factor antagonist modulates leukocyte trafficking and protects mouse livers against ischemia/reperfusion injury. Am J Pathol. 2006; 168:695–705. PMID: 16436682.
Article21. Takatsuka H, Takemoto Y, Okamoto T, Fujimori Y, Tamura S, Wada H, Okada M, Kanamaru A, Kakishita E. Adult respiratory distress syndrome-like disorders after allogeneic bone marrow transplantation. Transplantation. 1999; 68:1343–1347. PMID: 10573074.
Article22. Takatsuka H, Takemoto Y, Okamoto T, Fujimori Y, Tamura S, Wada H, Okada M, Kanamaru A, Kakishita E. Thrombotic microangiopathy following allogeneic bone marrow transplantation. Bone Marrow Transplant. 1999; 24:303–306. PMID: 10455370.
Article23. Takatsuka H, Takemoto Y, Okamoto T, Fujimori Y, Tamura S, Wada H, Okada M, Yamada S, Kanamaru A, Kakishita E. Predicting the severity of graft-versus-host disease from interleukin-10 levels after bone marrow transplantation. Bone Marrow Transplant. 1999; 24:1005–1007. PMID: 10556960.
Article24. Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000; 95:2754–2759. PMID: 10779417.
Article25. Holler E. Cytokines, viruses, and graft-versus-host disease. Curr Opin Hematol. 2002; 9:479–484. PMID: 12394168.
Article26. Brok HP, Heidt PJ, van der Meide PH, Zurcher C, Vossen JM. Interferon-gamma prevents graft-versus-host disease after allogeneic bone marrow transplantation in mice. J Immunol. 1993; 151:6451–6459. PMID: 8245478.27. Sykes M, Harty MW, Szot GL, Pearson DA. Interleukin-2 inhibits graft-versus-host disease-promoting activity of CD4+ cells while preserving CD4- and CD8-mediated graft-versus-leukemia effects. Blood. 1994; 83:2560–2569. PMID: 7909457.
Article28. Yang YG, Sergio JJ, Pearson DA, Szot GL, Shimizu A, Sykes M. Interleukin-12 preserves the graft-versus-leukemia effect of allogeneic CD8 T cells while inhibiting CD4-dependent graft-versus-host disease in mice. Blood. 1997; 90:4651–4660. PMID: 9373279.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chronic graft versus host disease with small bowel obstruction after unrelated hematopoietic stem cell transplantation in a patient with acute myeloid leukemia
- A case of pneumomediastinum combined with chronic graft-versus-host disease following allogeneic hematopoietic stem cell transplantation
- The Strategies for the Prevention of Chronic GVHD in Hematopoietic Stem Cell Transplantation
- Distribution of CD4+CD25+ T cells and graft-versus-host disease in human hematopoietic stem cell transplantation
- Enhancement of Graft-versus-leukemia Effects by Mesenchymal Stem Cells in Mixed Chimerisim after a Murine Non-myeloablative Hematopoietic Stem Cell Transplantation